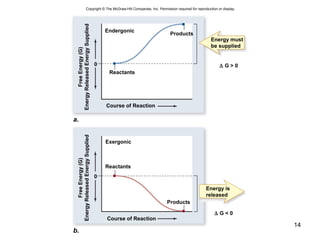
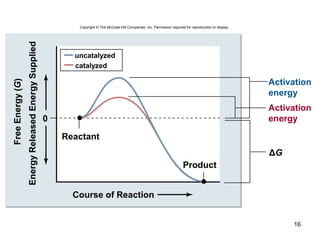
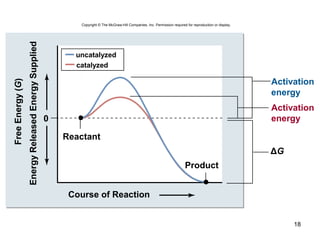
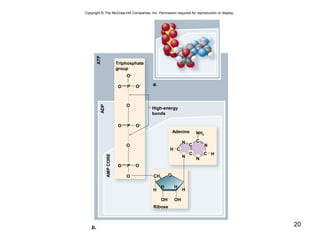
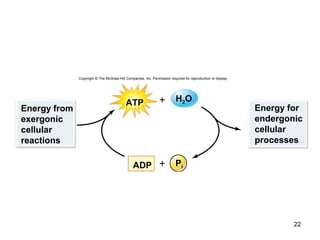
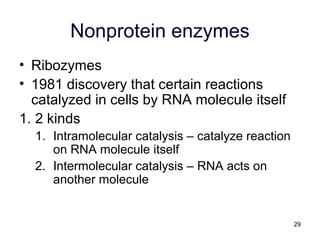
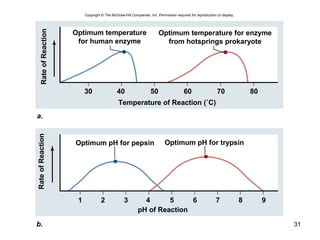
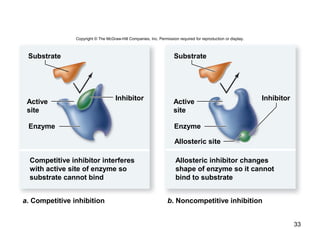
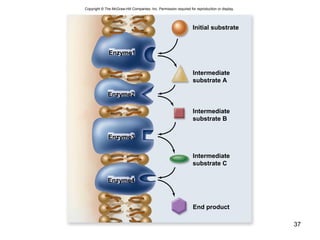
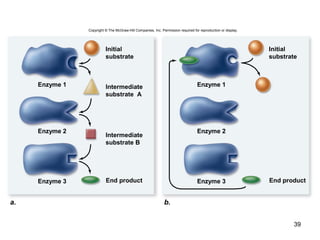
This document provides an outline for a lecture on energy and metabolism. It begins with definitions of key thermodynamic concepts like energy, redox reactions, and the laws of thermodynamics. It then discusses how cells use ATP as a currency for energy transfer and storage. Enzymes are introduced as biological catalysts that lower the activation energy of reactions. Various factors that influence enzyme function and inhibition are also covered. Finally, the document outlines biochemical pathways and feedback inhibition as a means of regulating metabolic pathways.