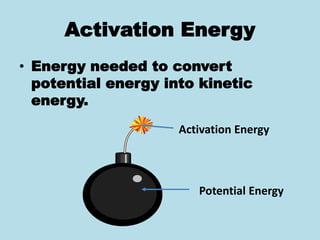
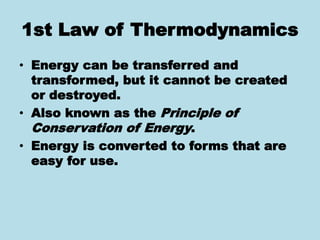
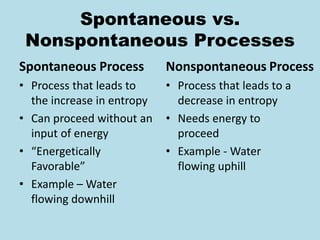
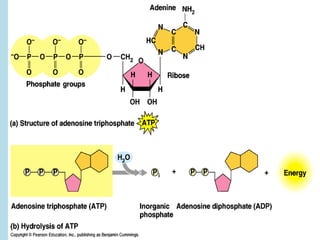
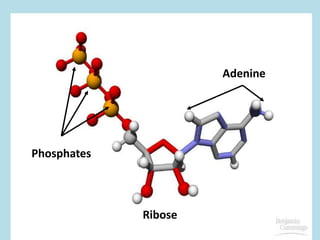
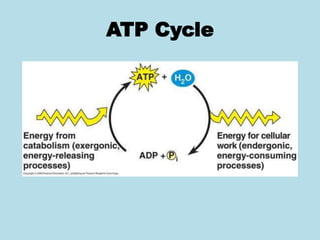
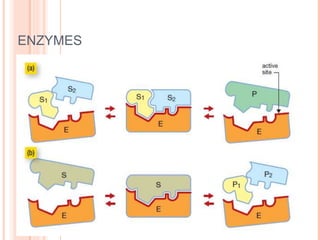
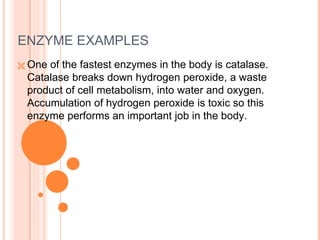
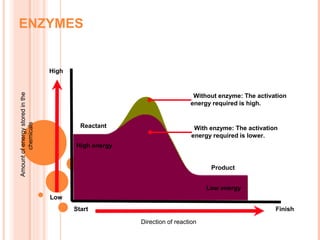
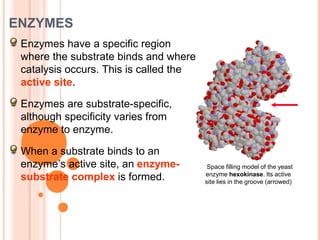
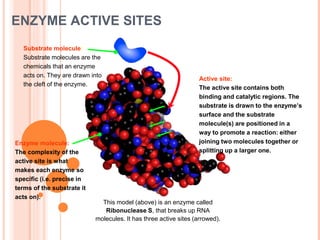
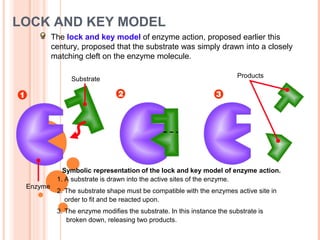
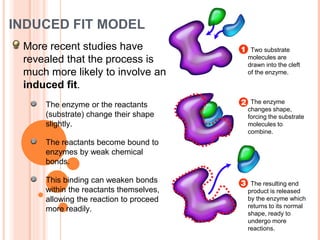
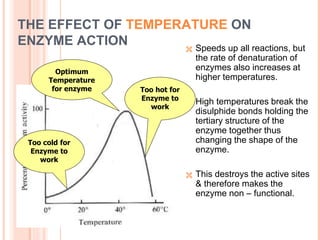
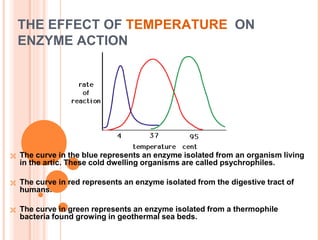
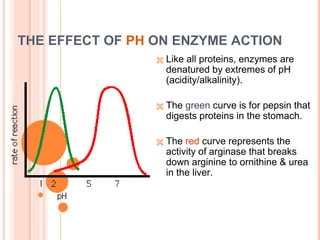
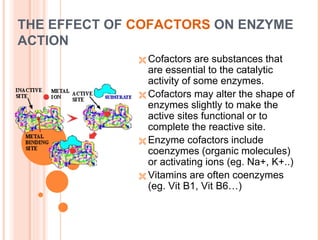
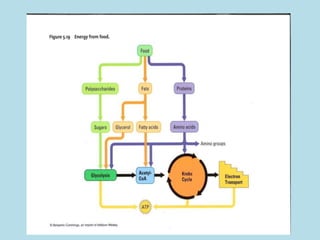
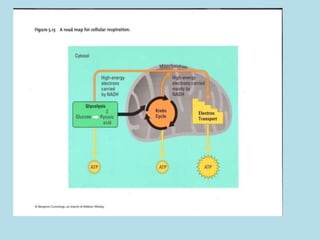
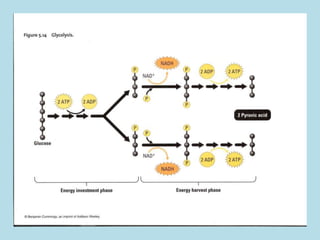
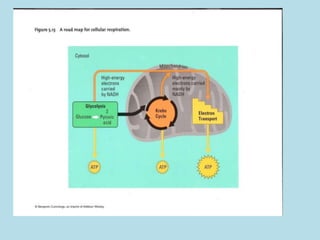
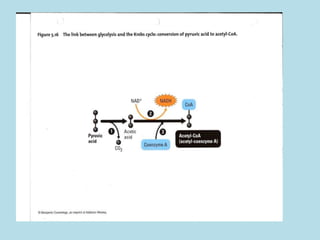
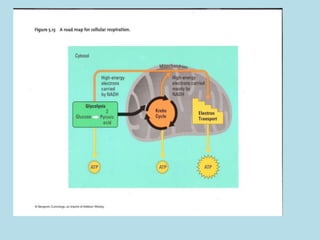
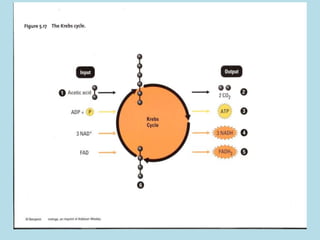
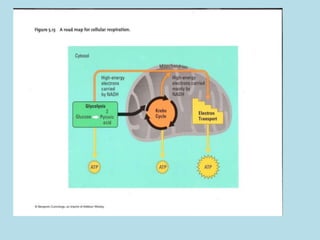
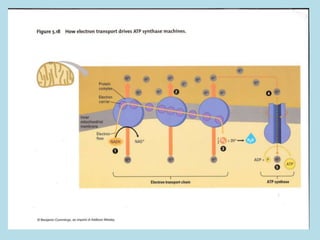
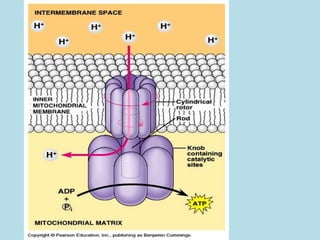
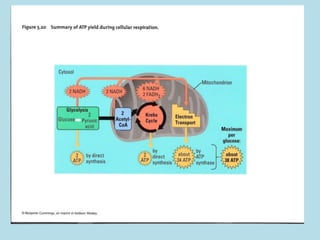
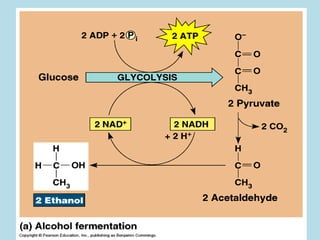
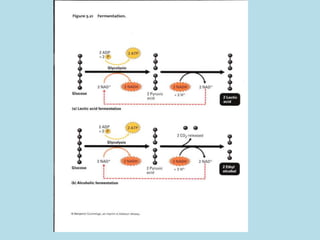
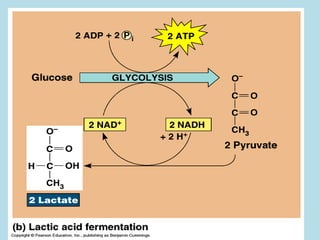
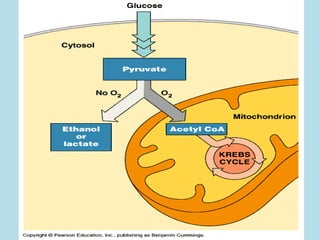
Metabolism involves the chemical processes that take place in organisms. There are two main types of metabolic pathways - catabolic pathways that break down molecules and release energy, and anabolic pathways that use energy to build molecules. Cellular respiration uses catabolic pathways to break down glucose and other food molecules, releasing energy that is captured in ATP. There are four main stages of cellular respiration: glycolysis, pyruvate oxidation, the citric acid cycle, and the electron transport chain. When oxygen is absent, cells carry out anaerobic respiration like lactic acid fermentation or alcoholic fermentation to regenerate NAD+ and allow glycolysis to continue producing a small amount of ATP.