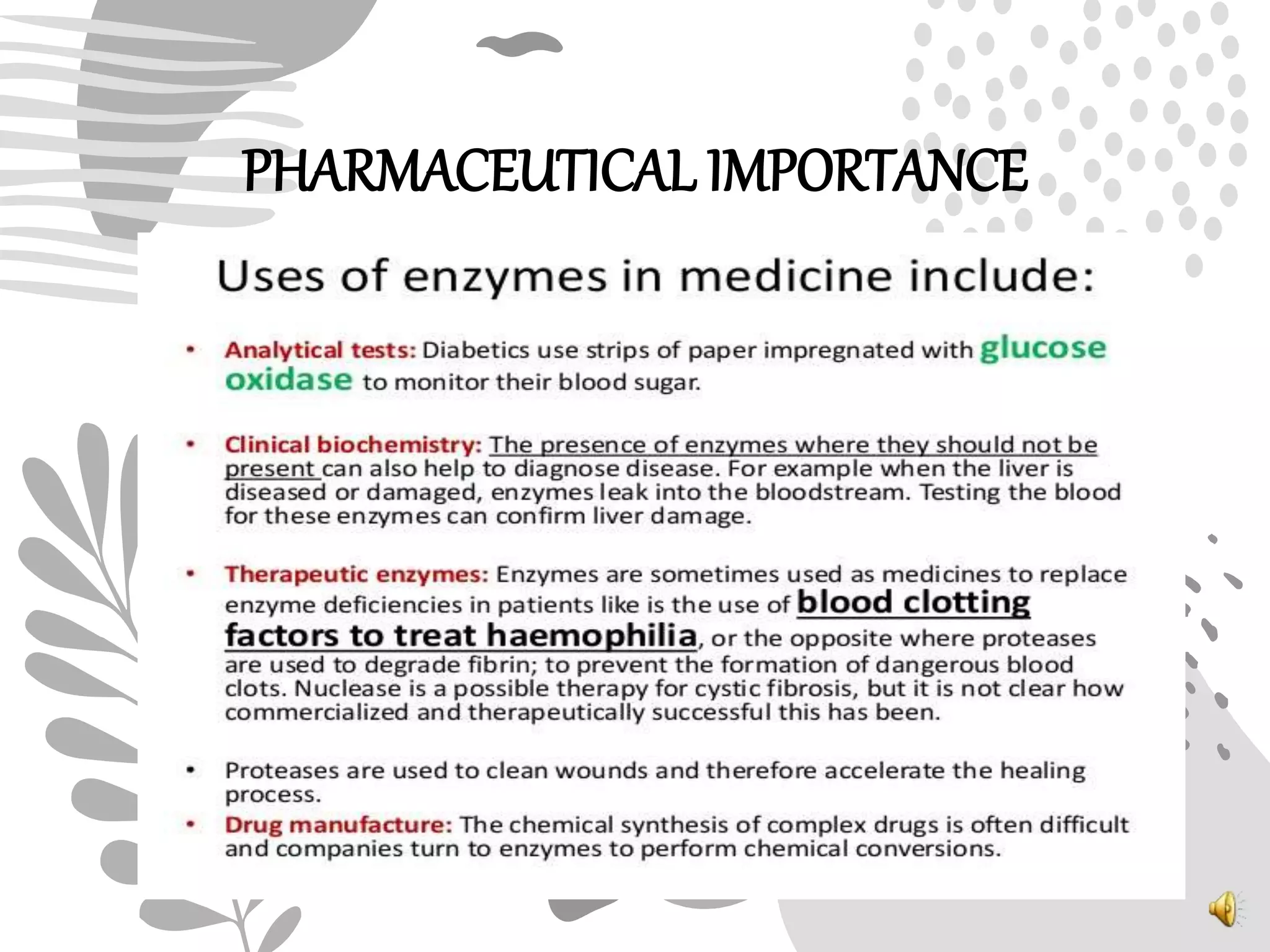
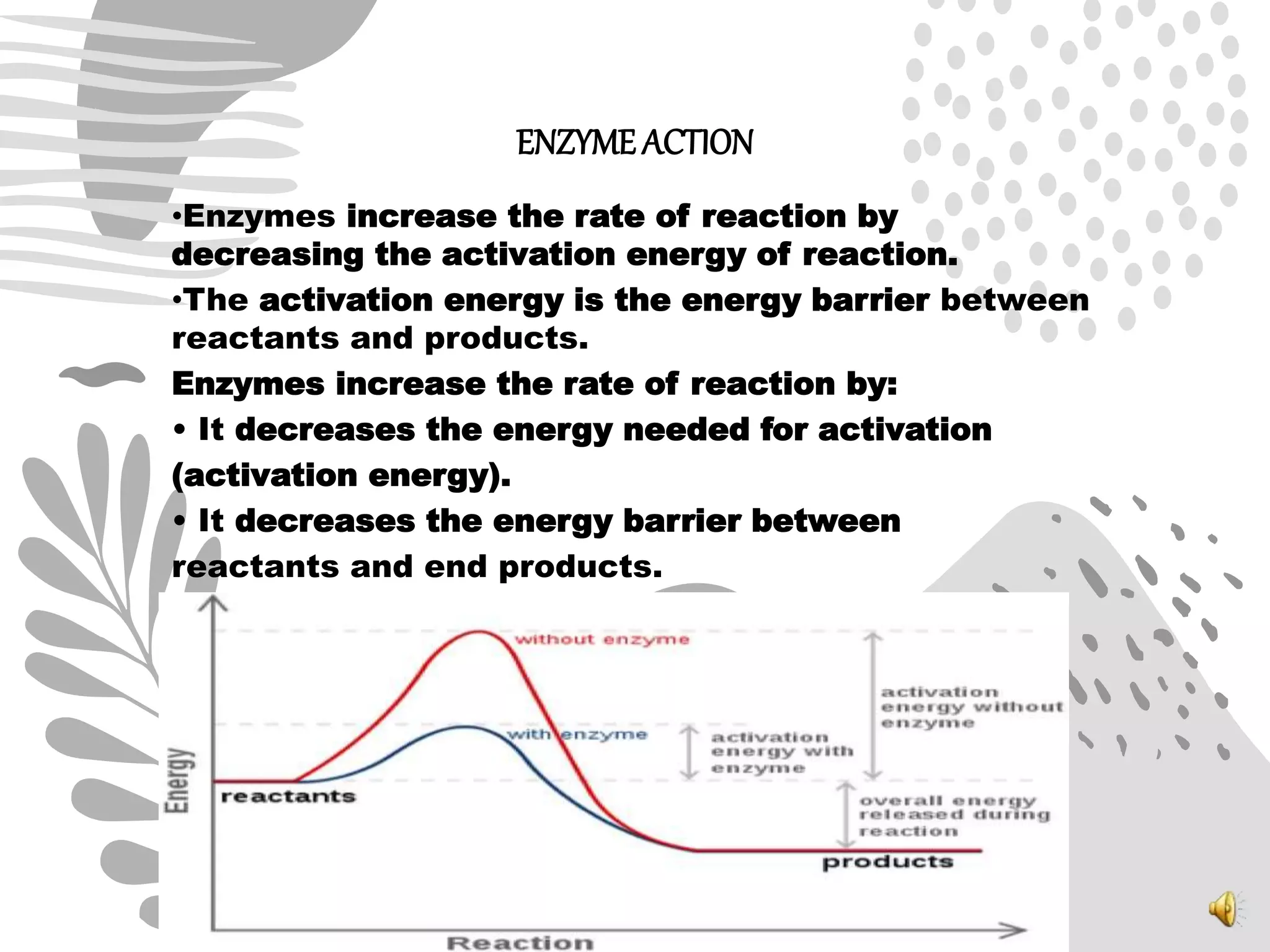
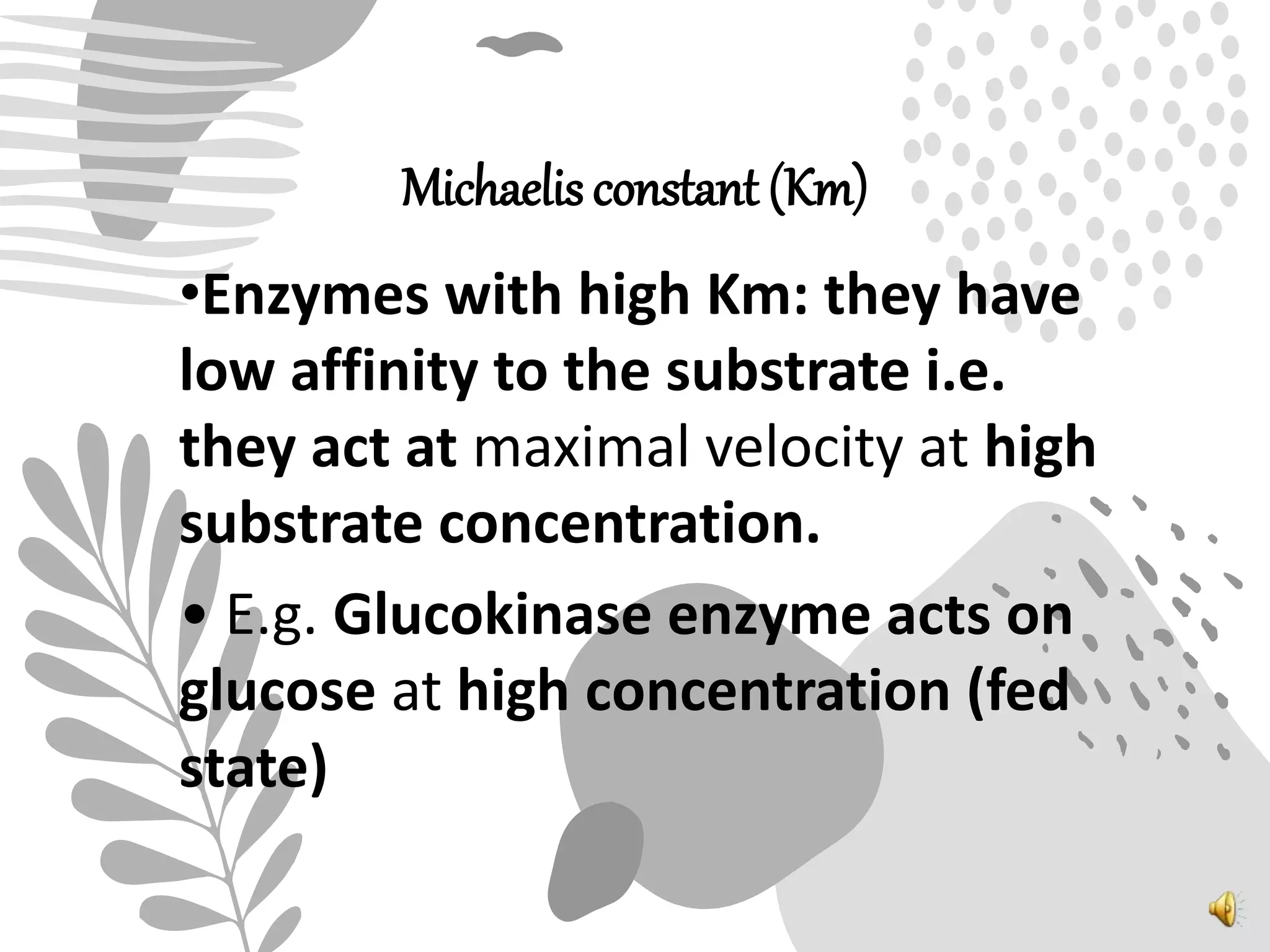
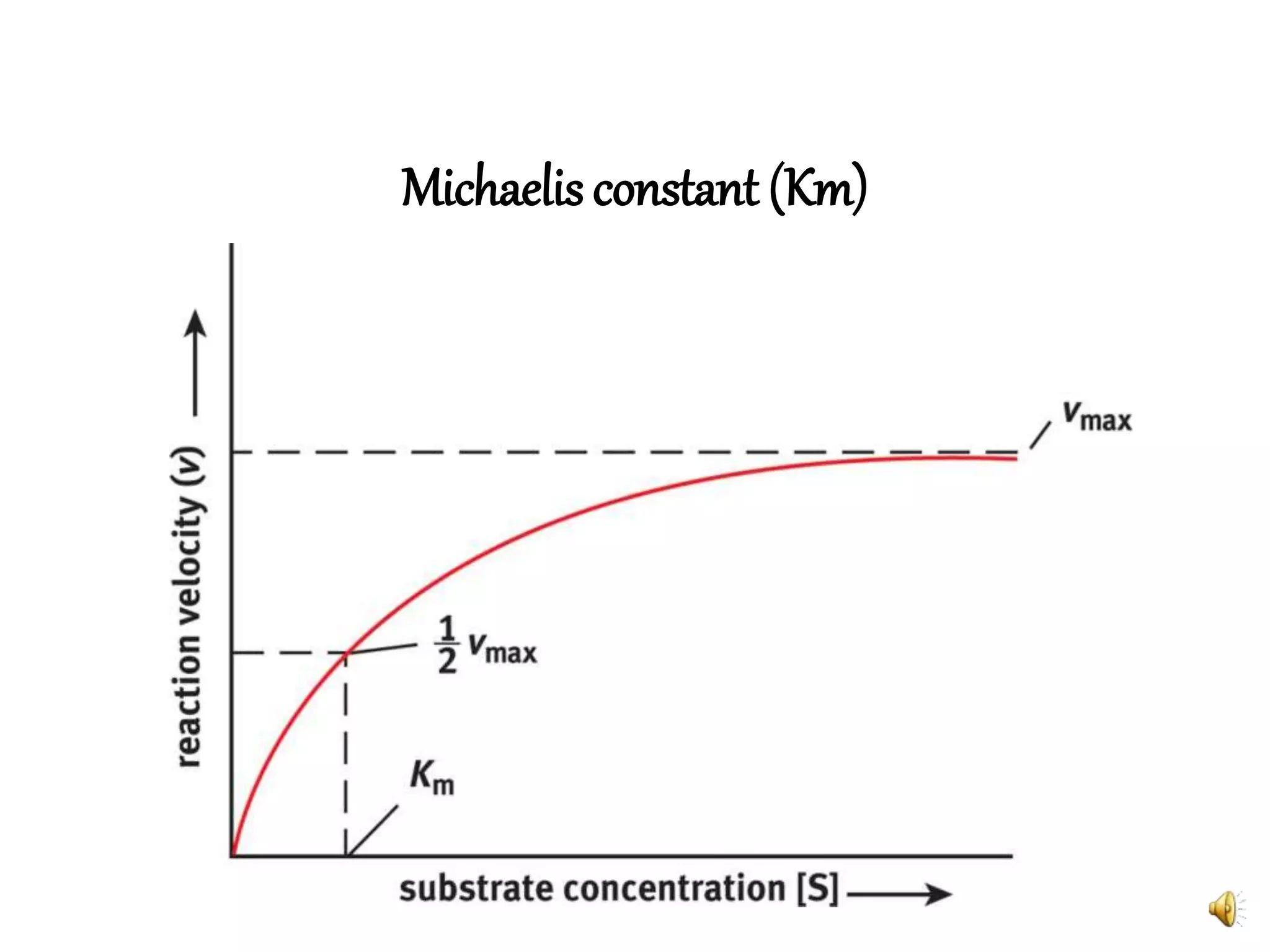
1. Enzymes catalyze biochemical reactions by lowering the activation energy between reactants and products, increasing reaction rates.
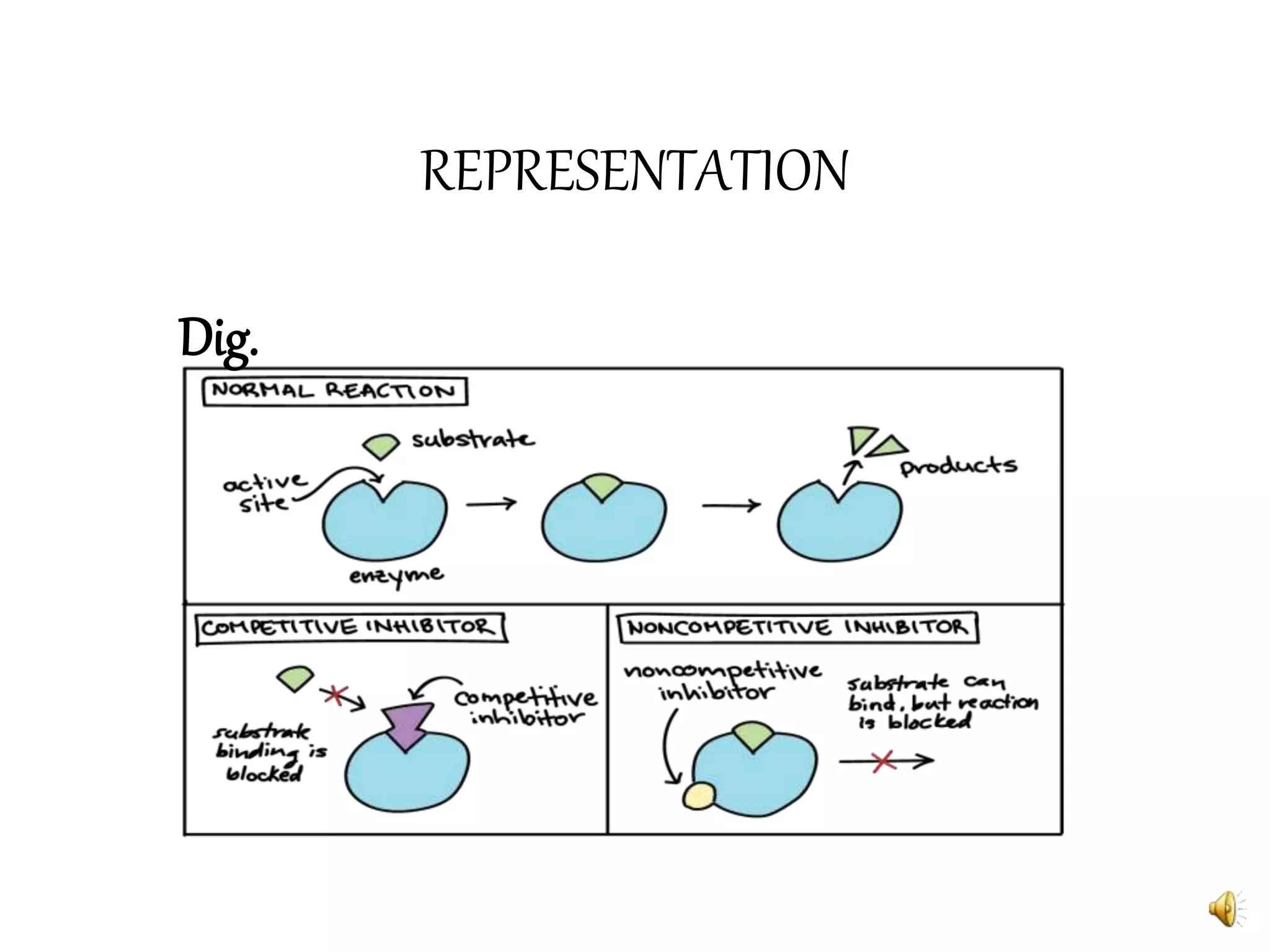
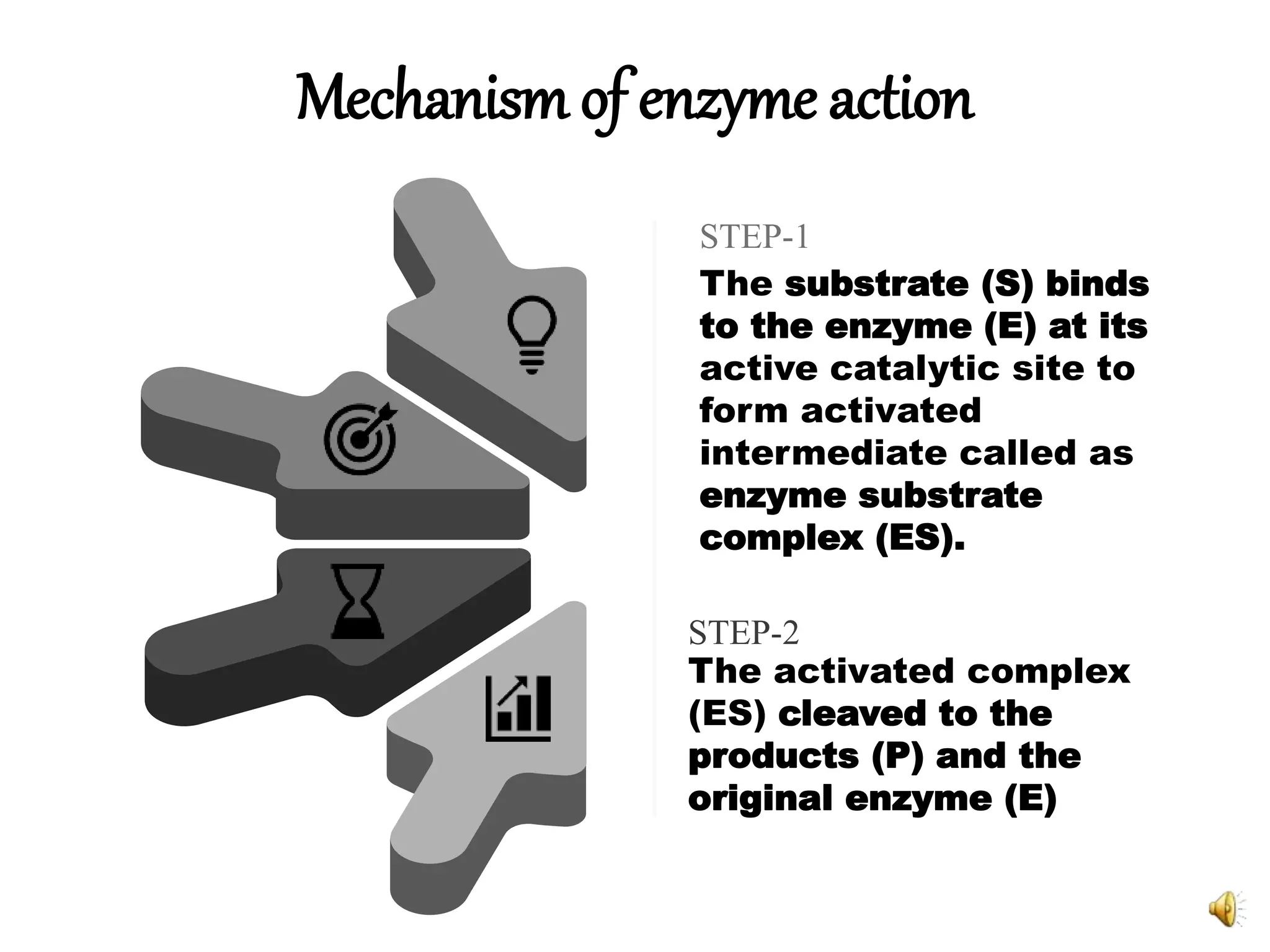
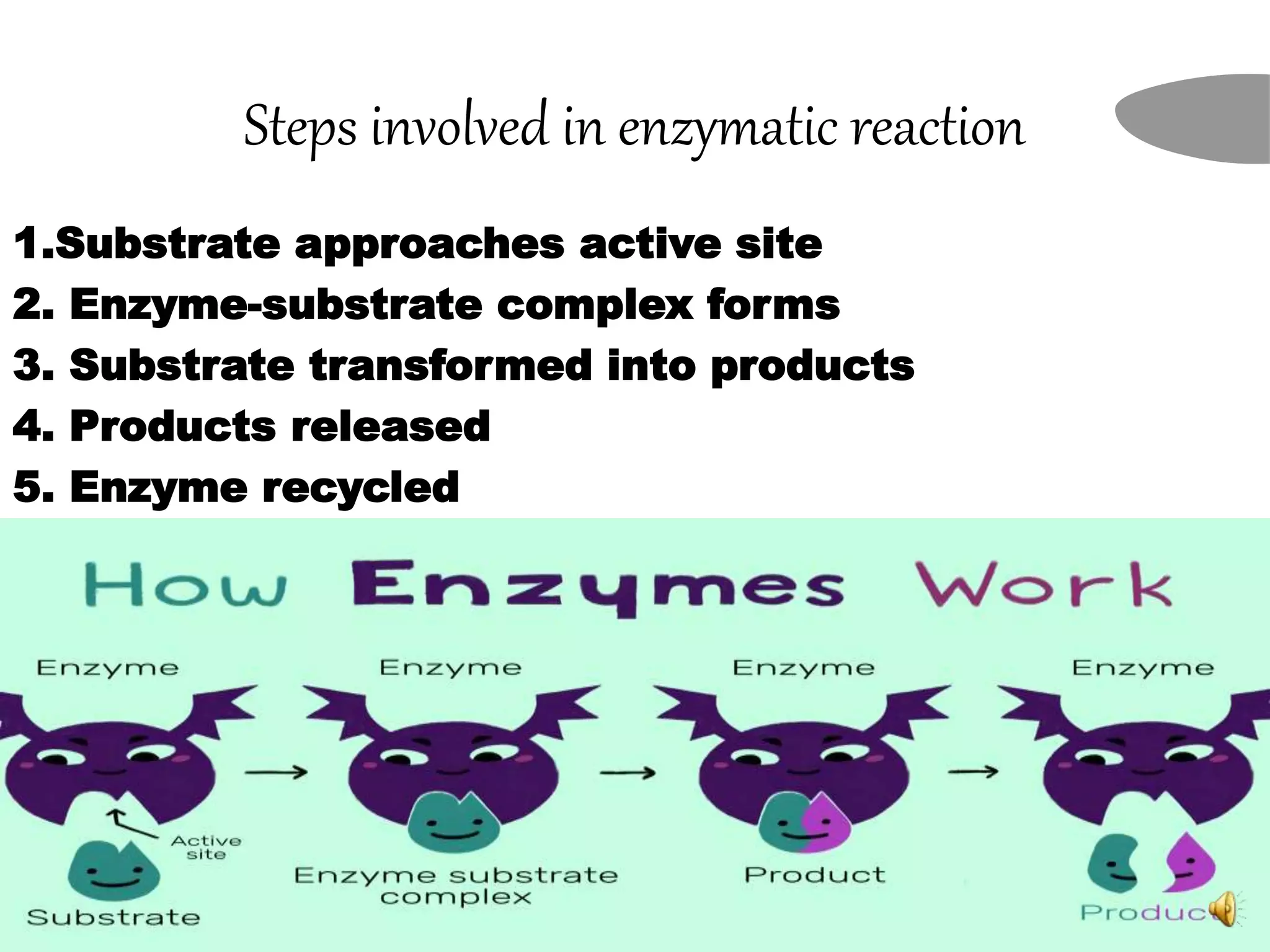
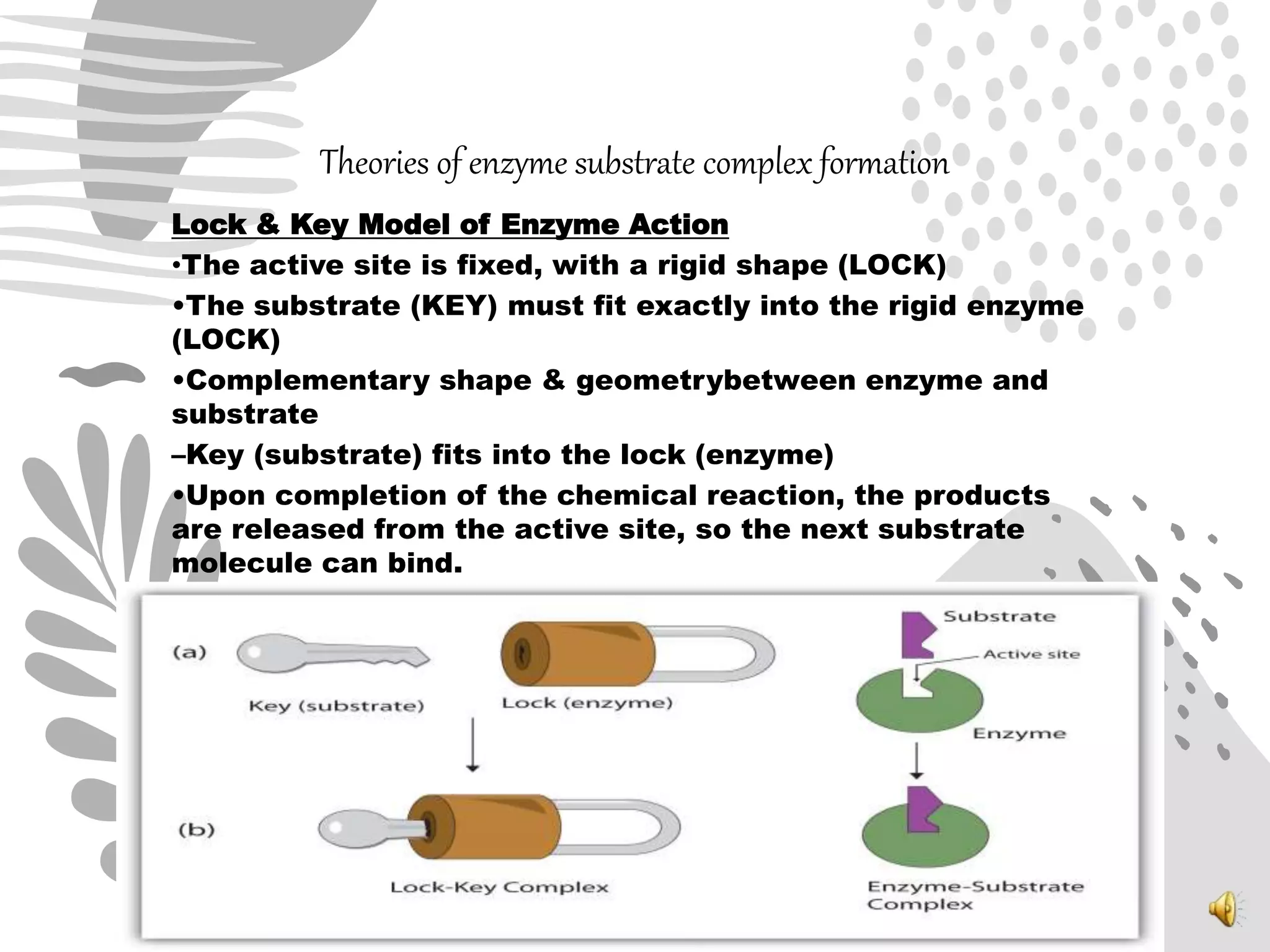
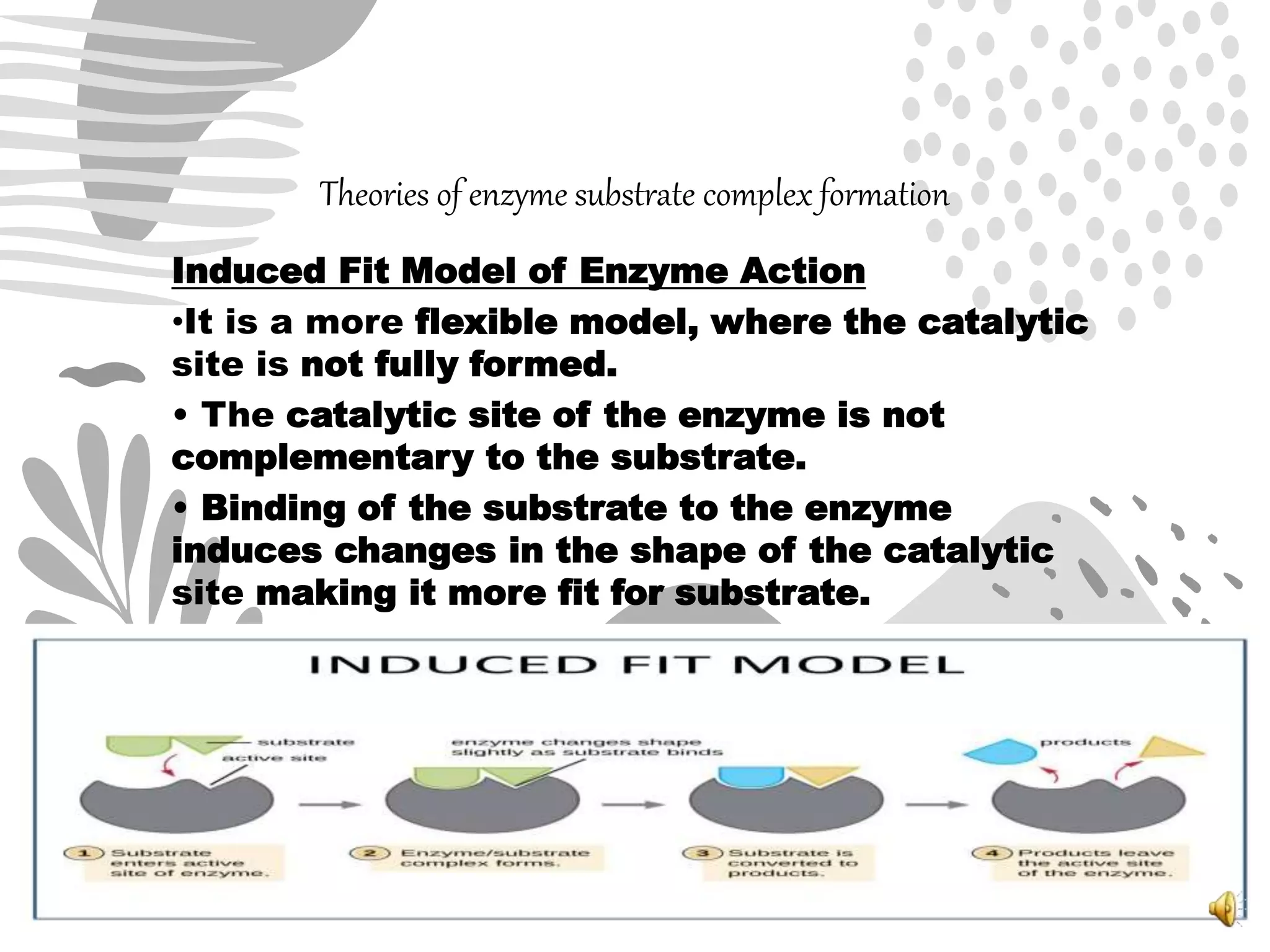
2. The mechanism of enzyme action involves substrate binding to the active site of the enzyme to form an enzyme-substrate complex, which then undergoes a reaction to form products and release the original enzyme.
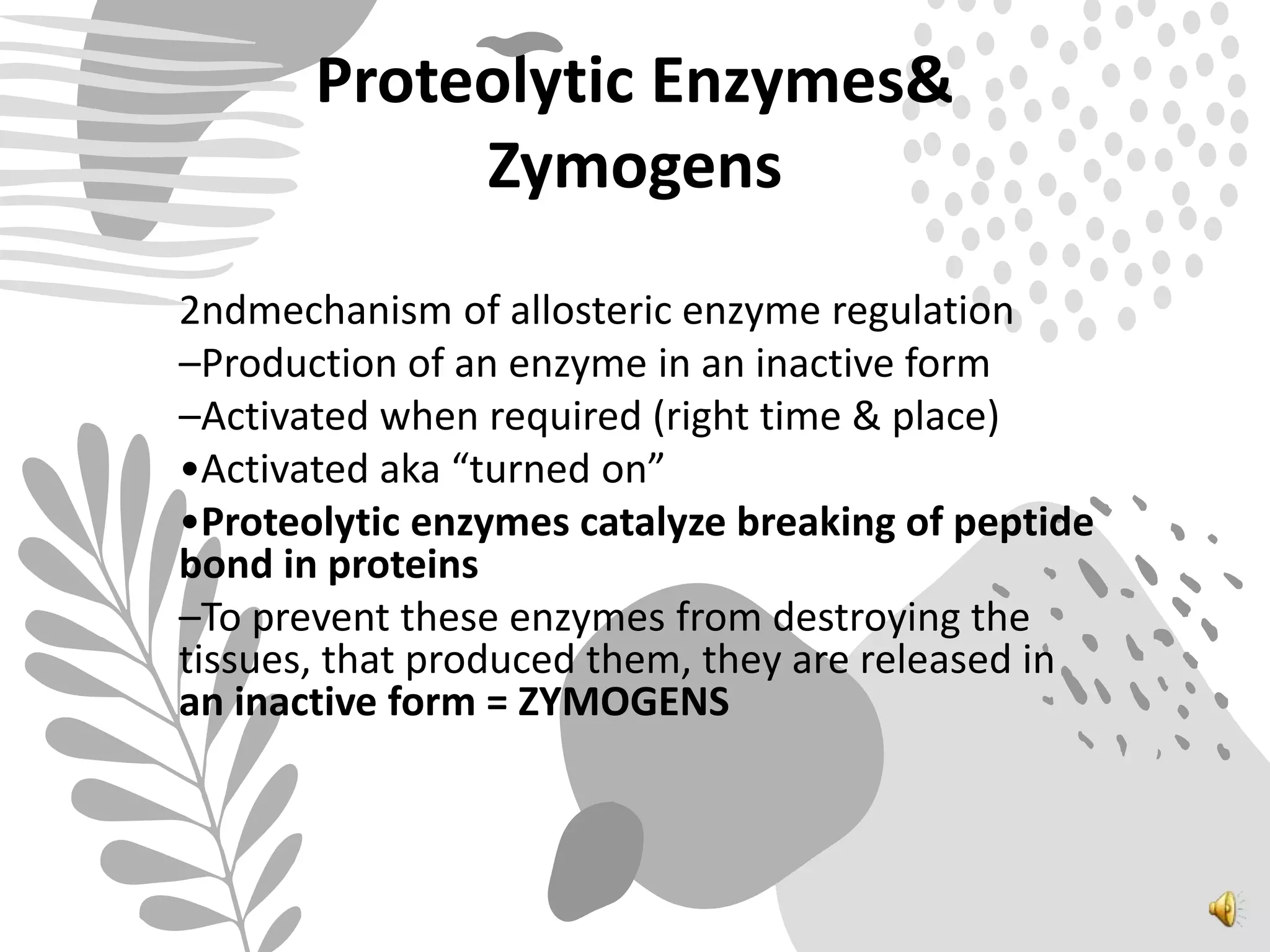
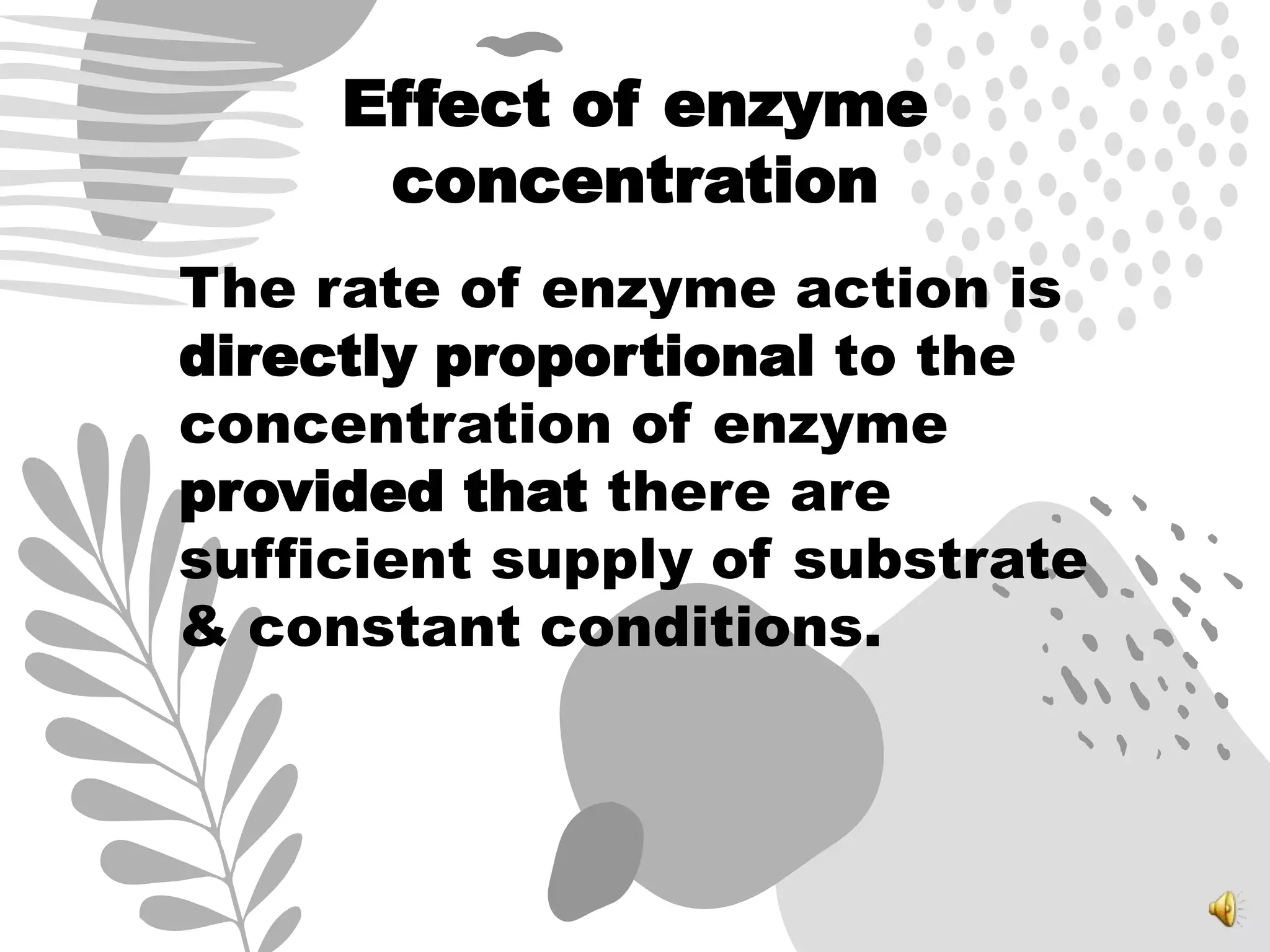
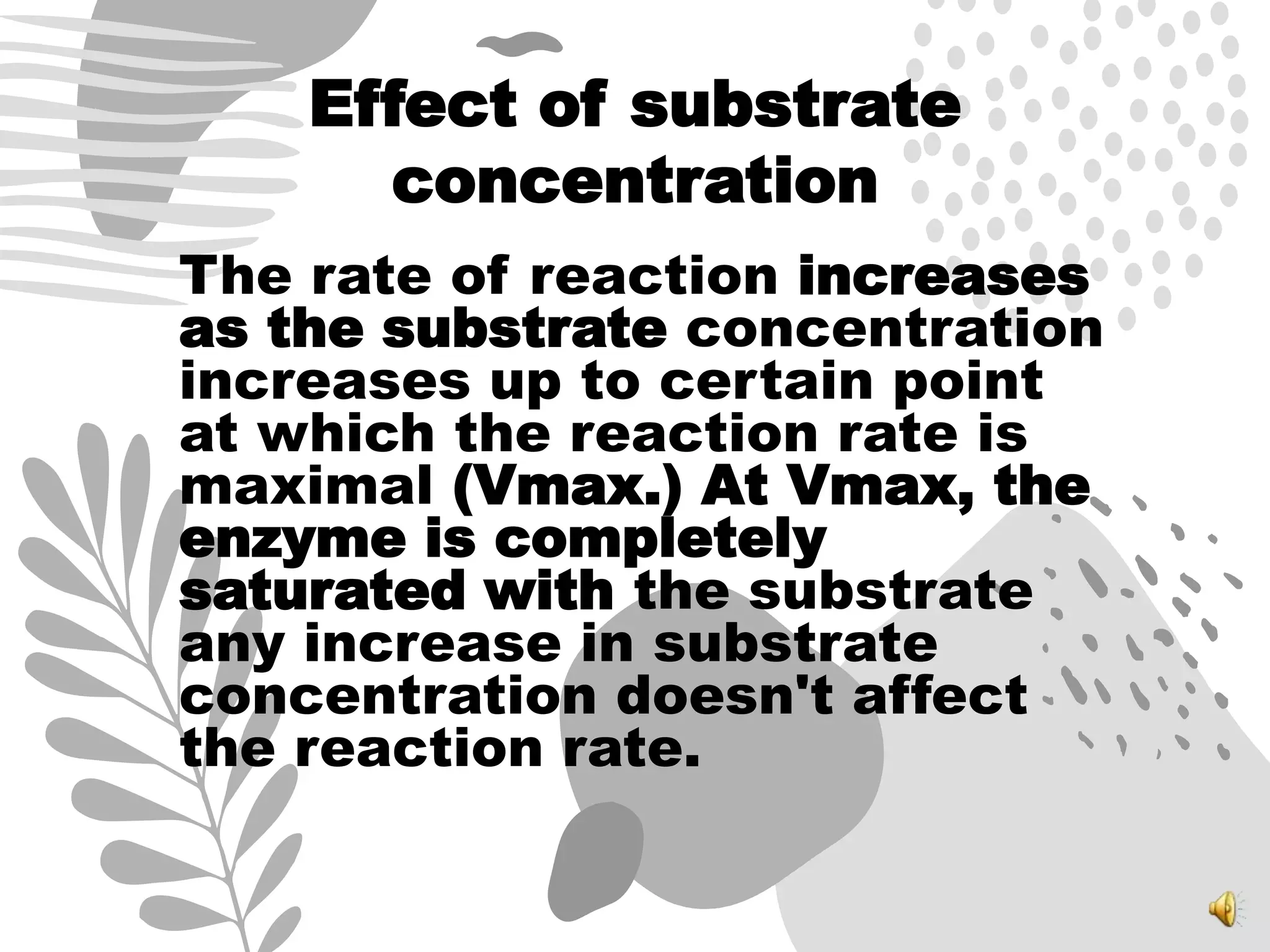
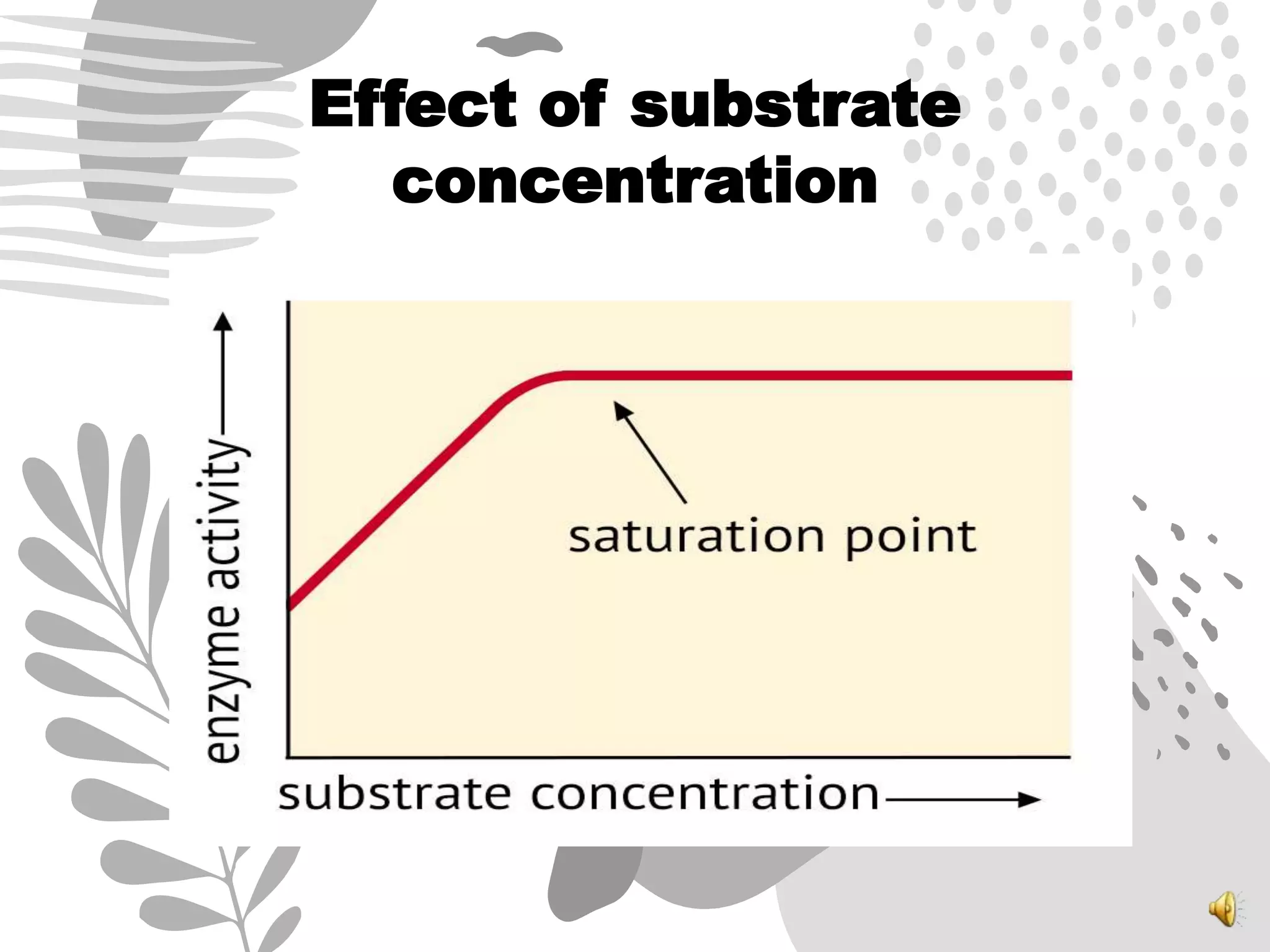
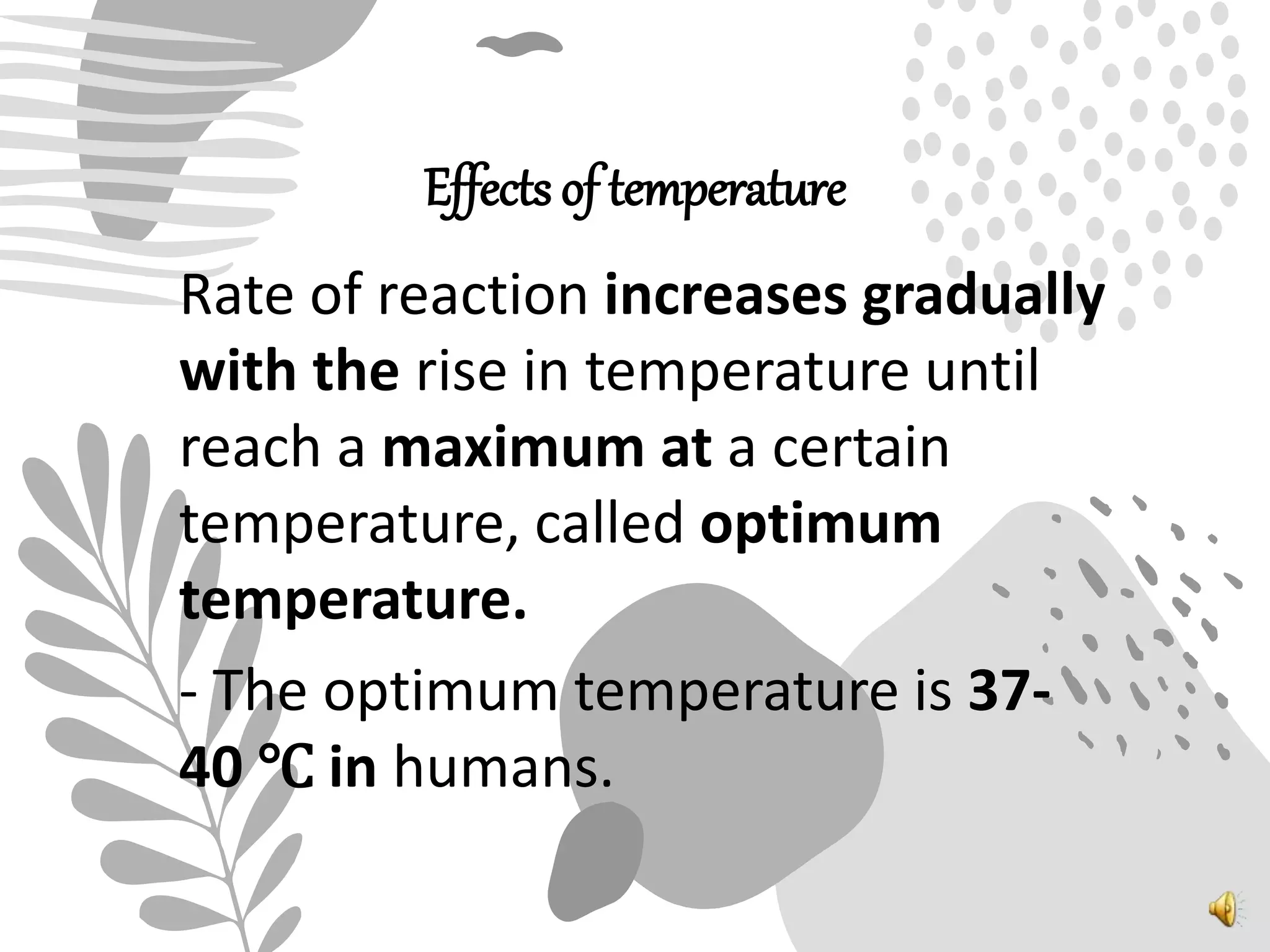
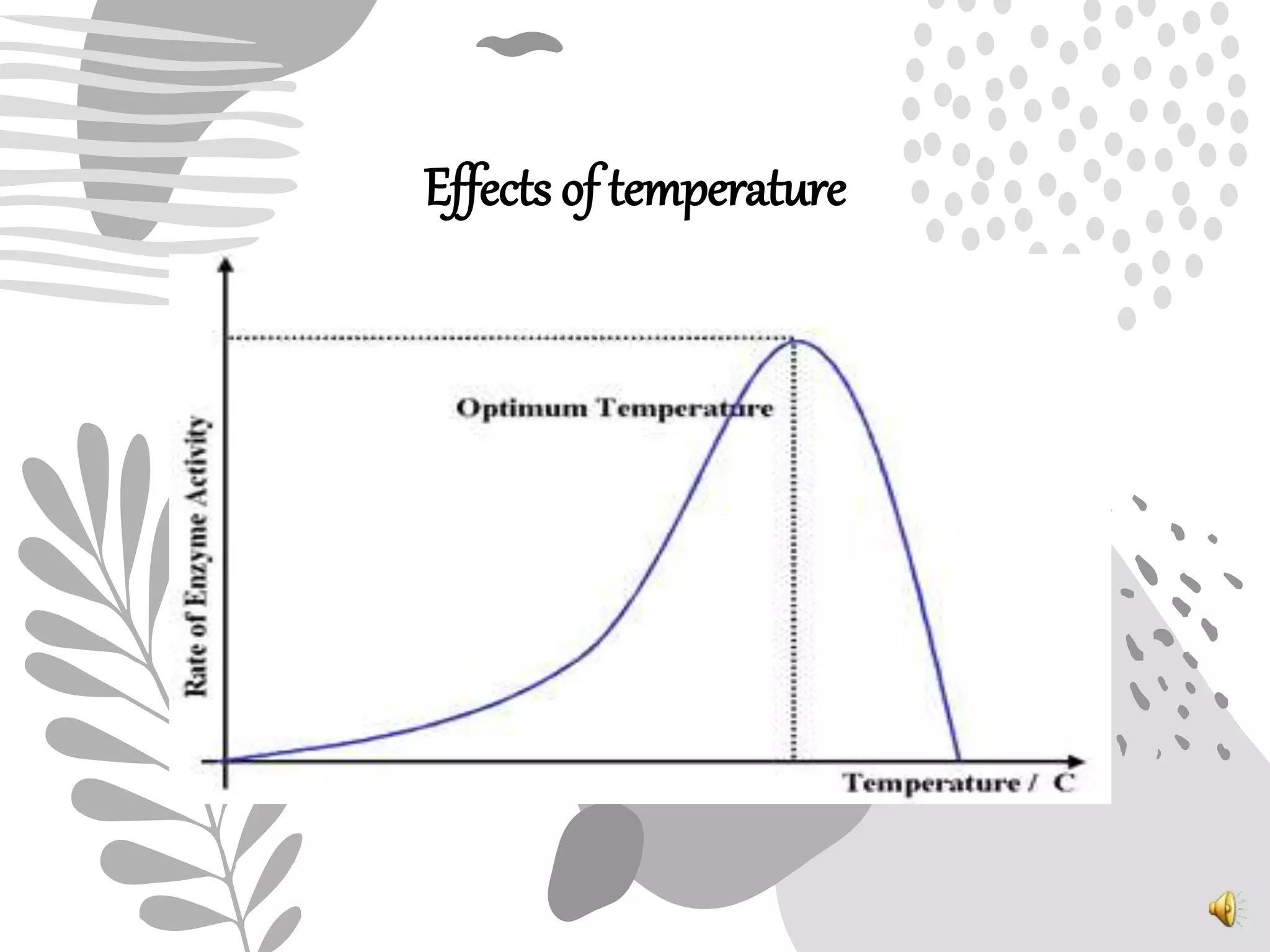
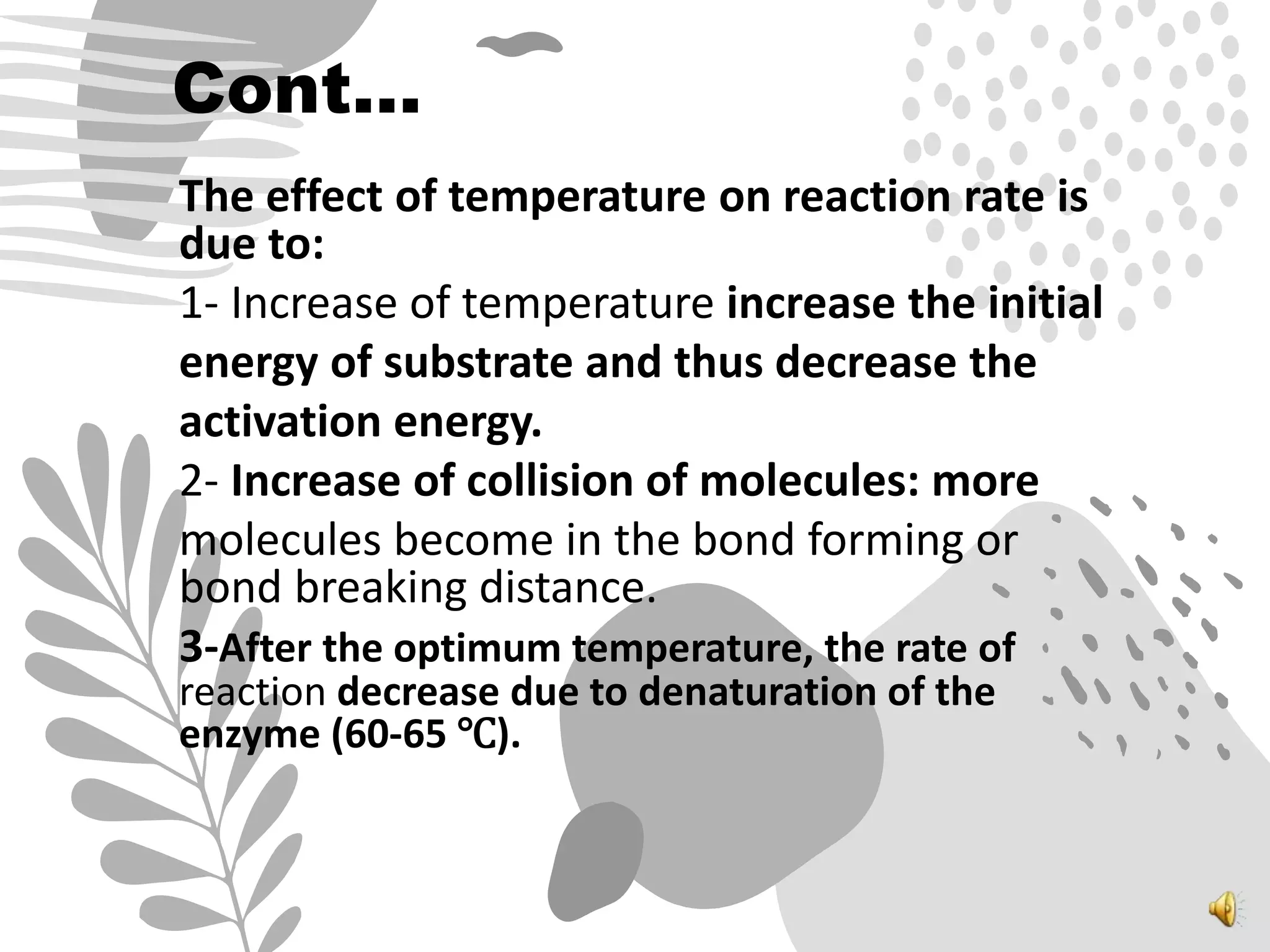
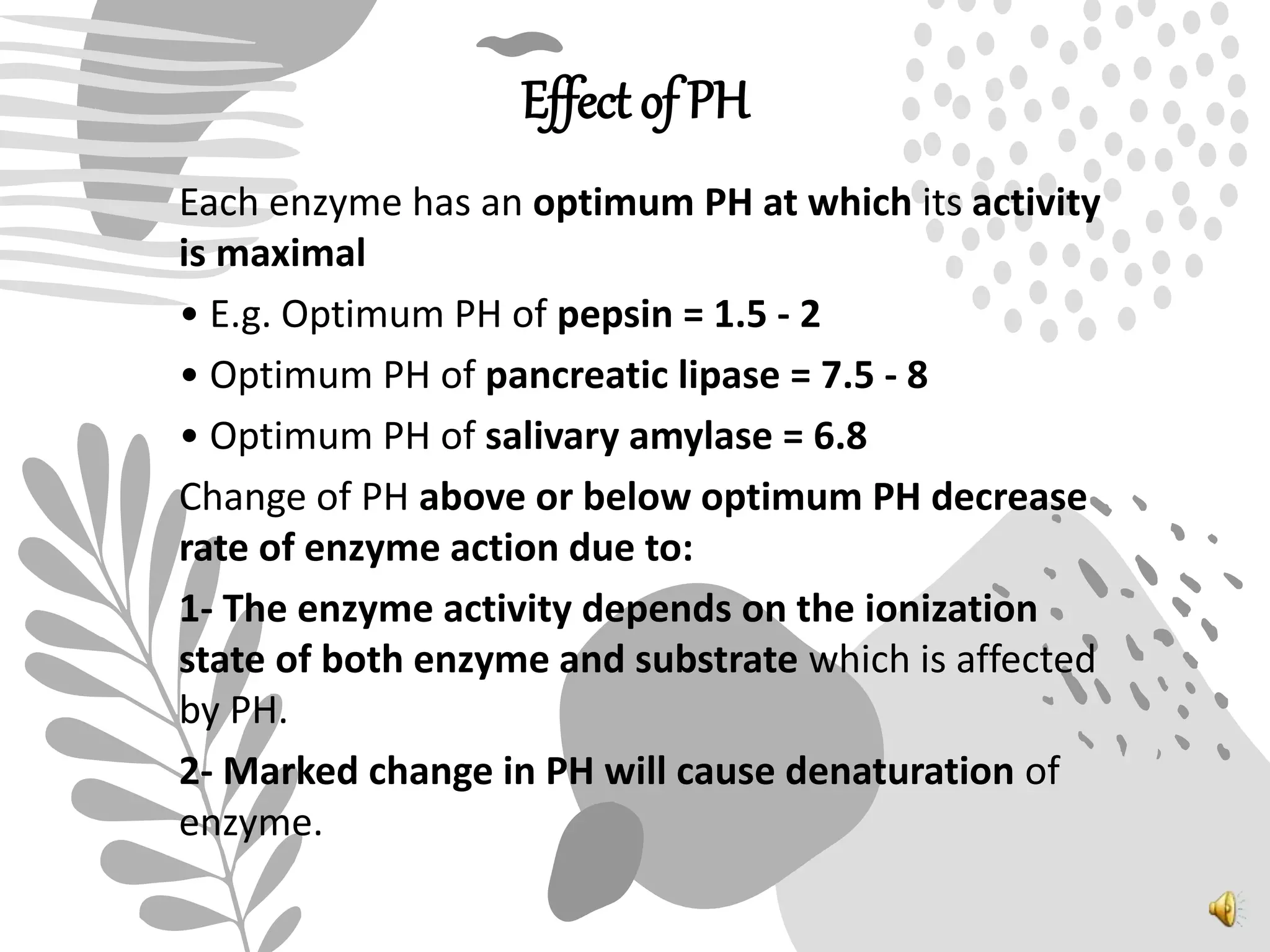
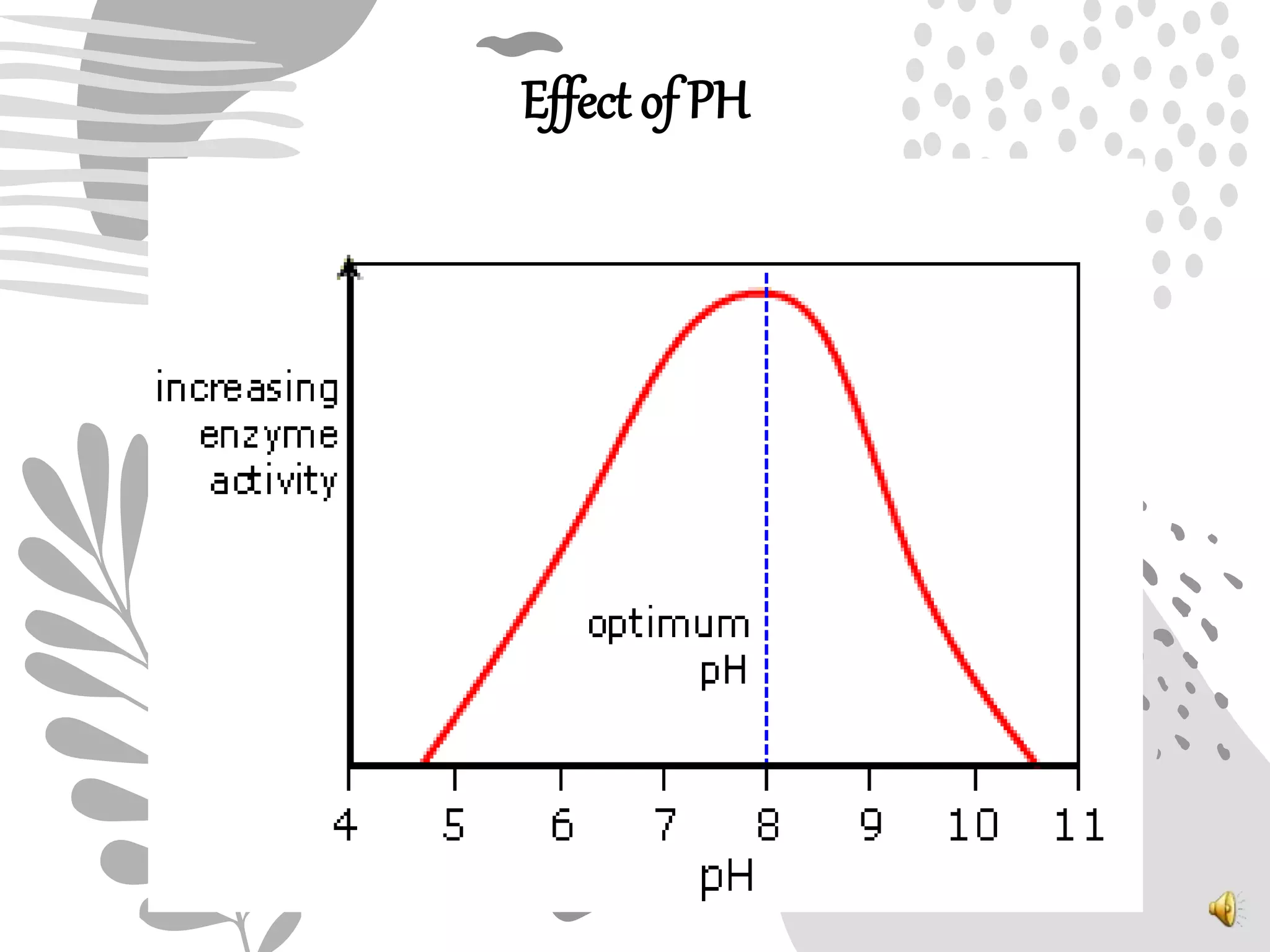
3. Reaction rates are affected by factors like temperature, pH, substrate and enzyme concentrations, and the presence of inhibitors or activators that can bind to the enzyme and alter its structure and activity.







![Reversible Noncompetitive
Inhibition
A non-competitive inhibitor decreases enzyme activity by
binding to a site on the enzyme other than the active site
–The non-competitive inhibitor alters the tertiary structure of
the enzyme & the active site
•Decreasing enzyme activity
•Substrate cannot fit into active site
–Process can be reversed only by lowering the [non-competitive
inhibitor]
•Example:
–Heavy metals Pb2+& Hg2+bind to –SH of Cysteine, away from
active site
•Disrupt the secondary & tertiary structure](https://image.slidesharecdn.com/enzymespart-2-200531043933/75/Enzymes-Biochemistry-part-2-24-2048.jpg)