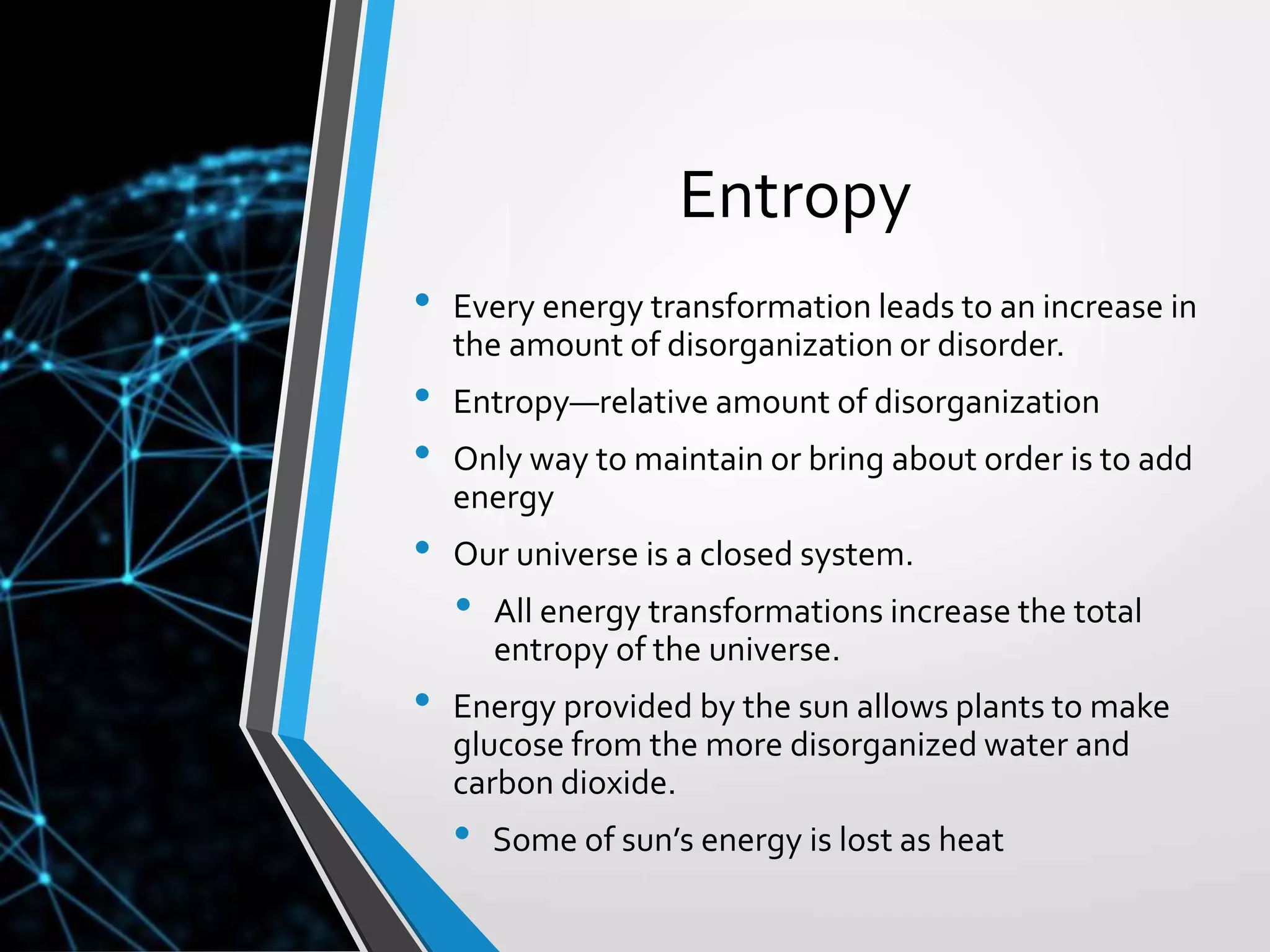
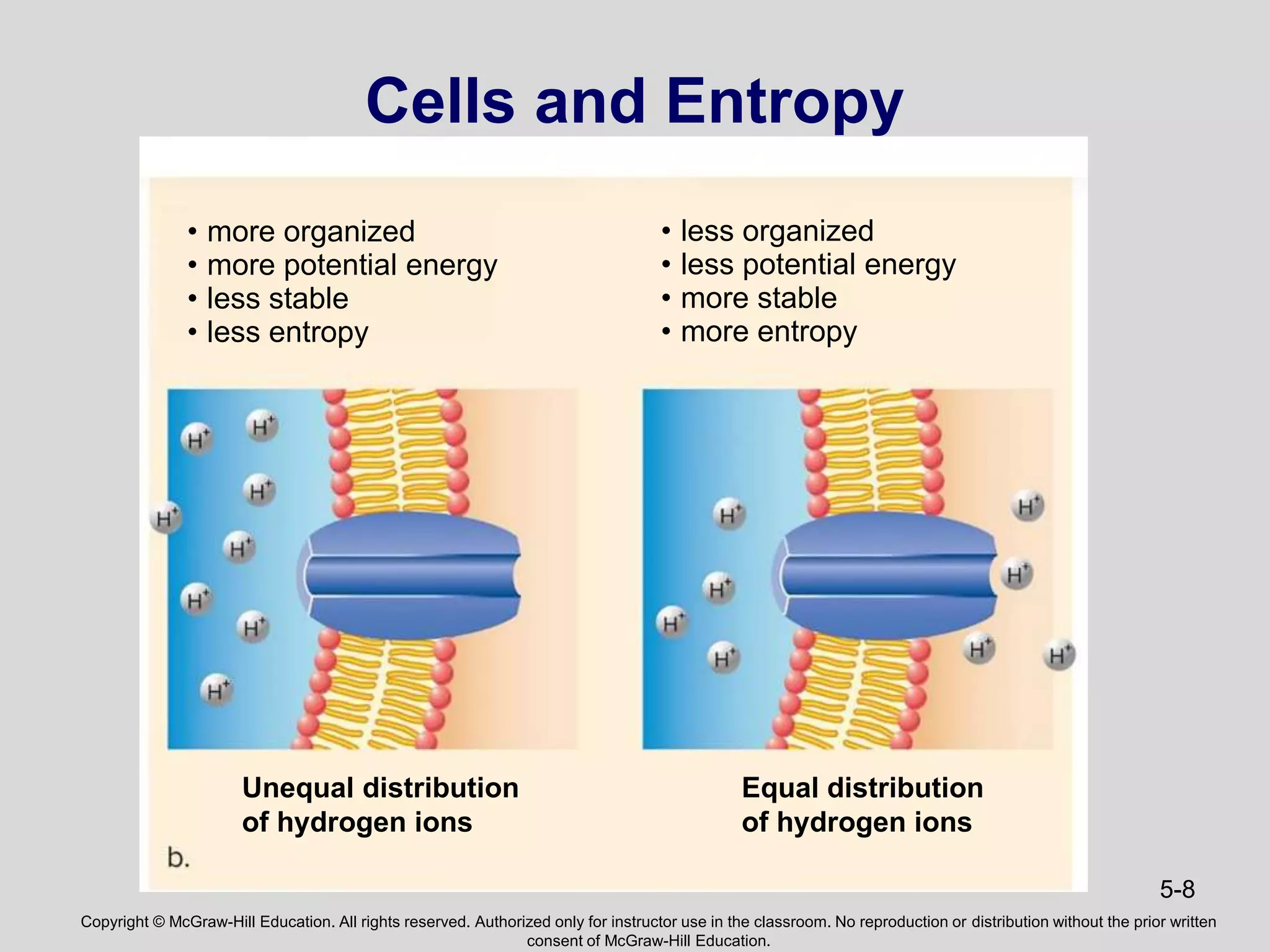
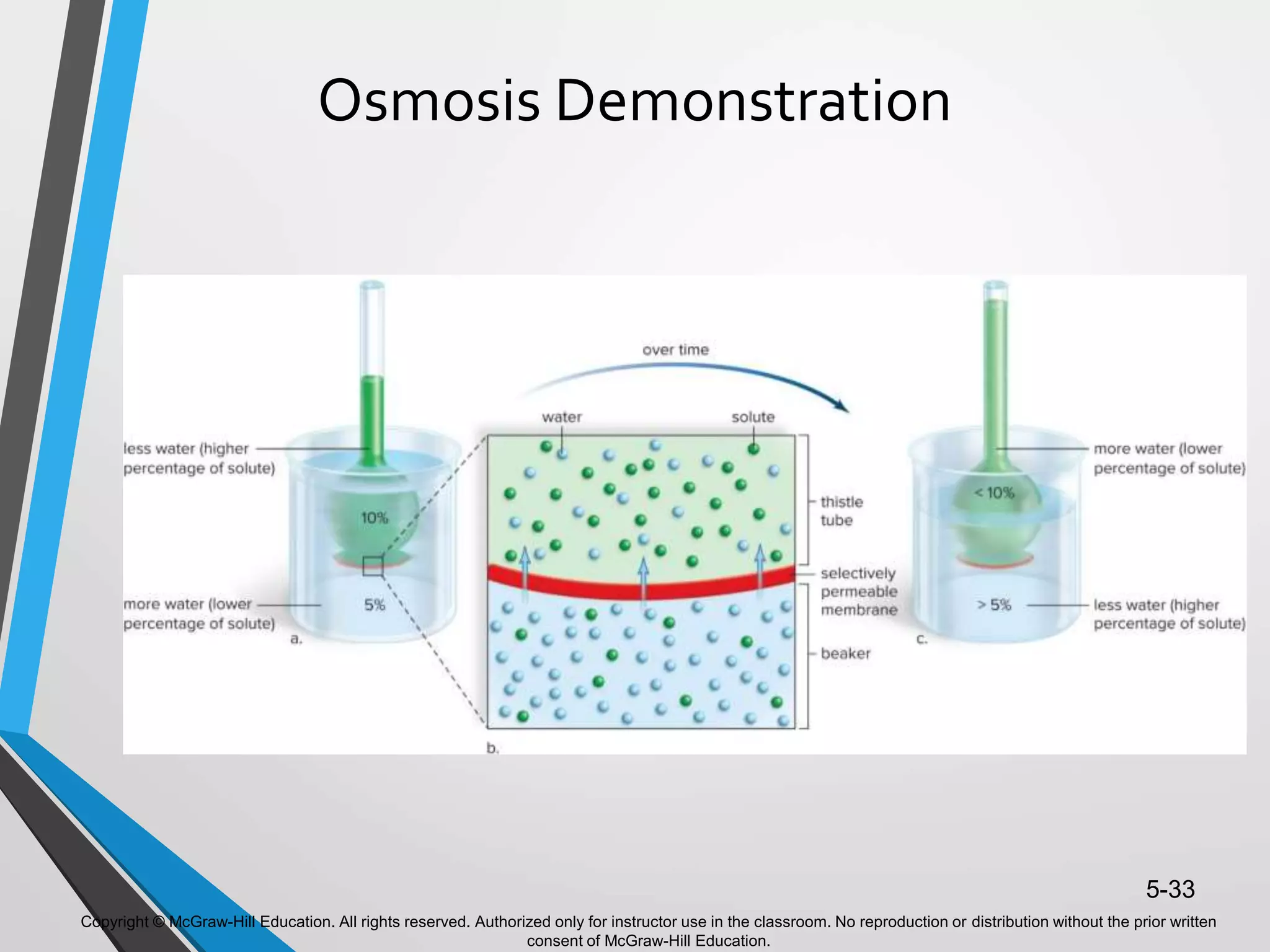
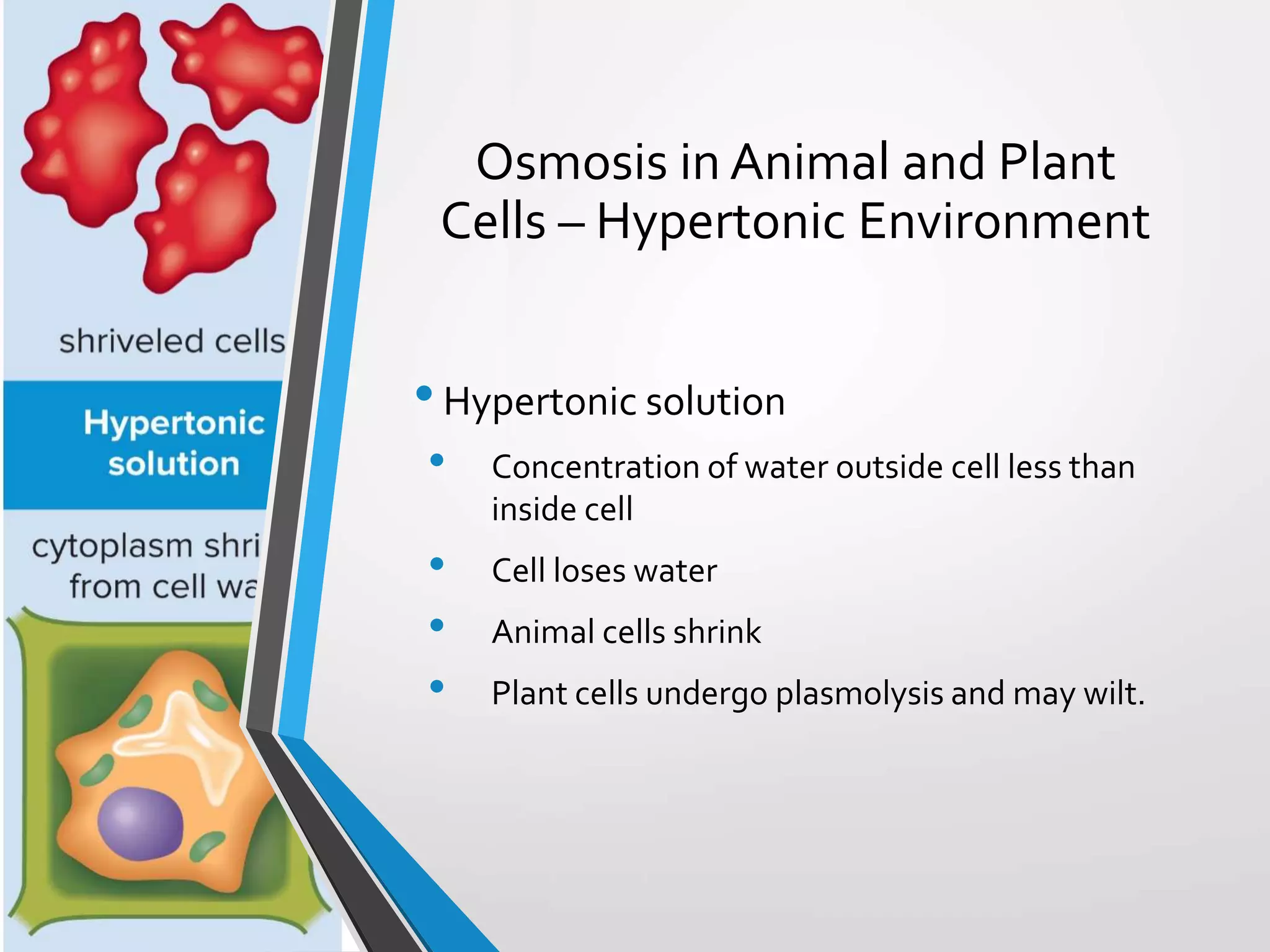
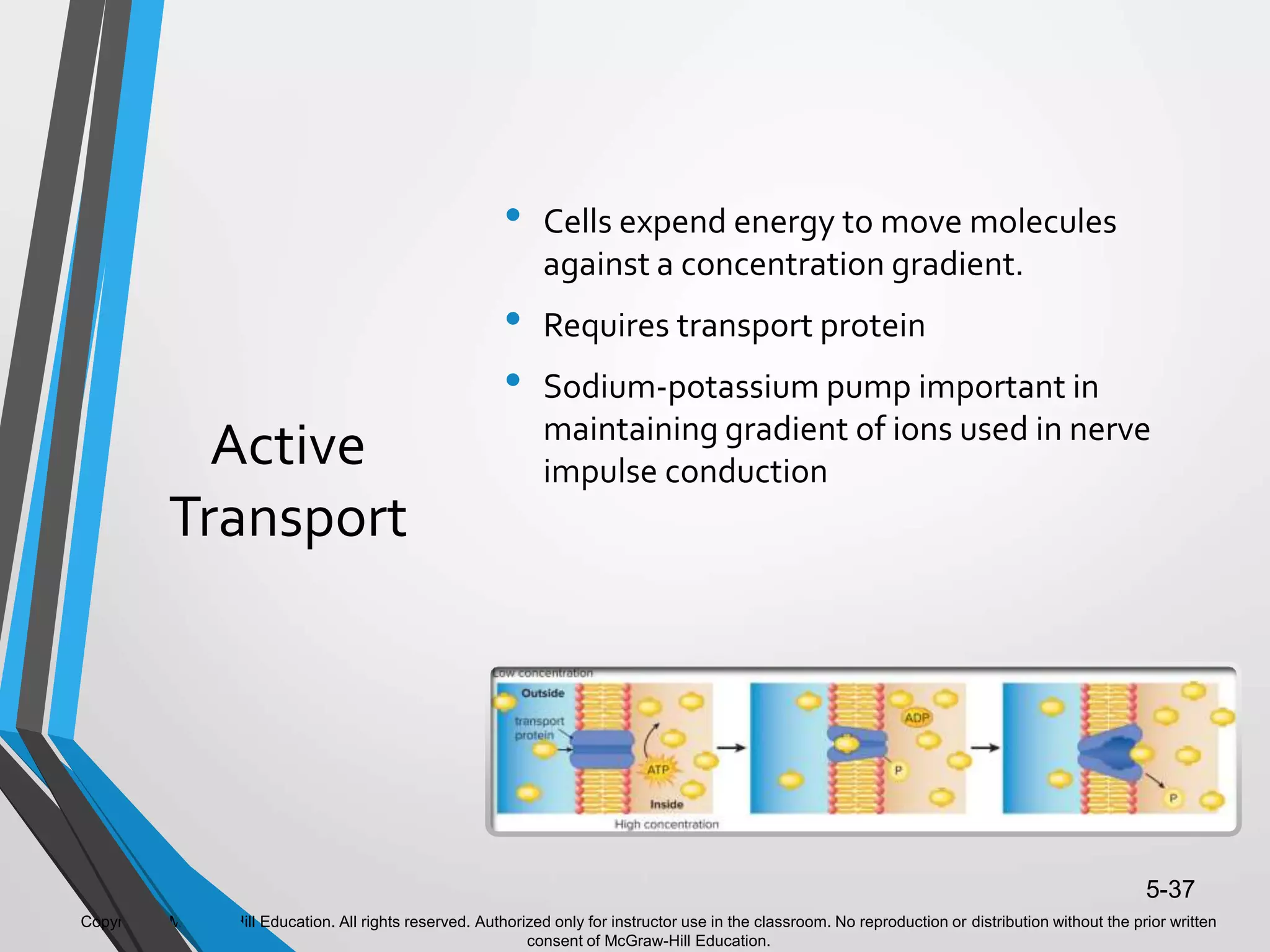
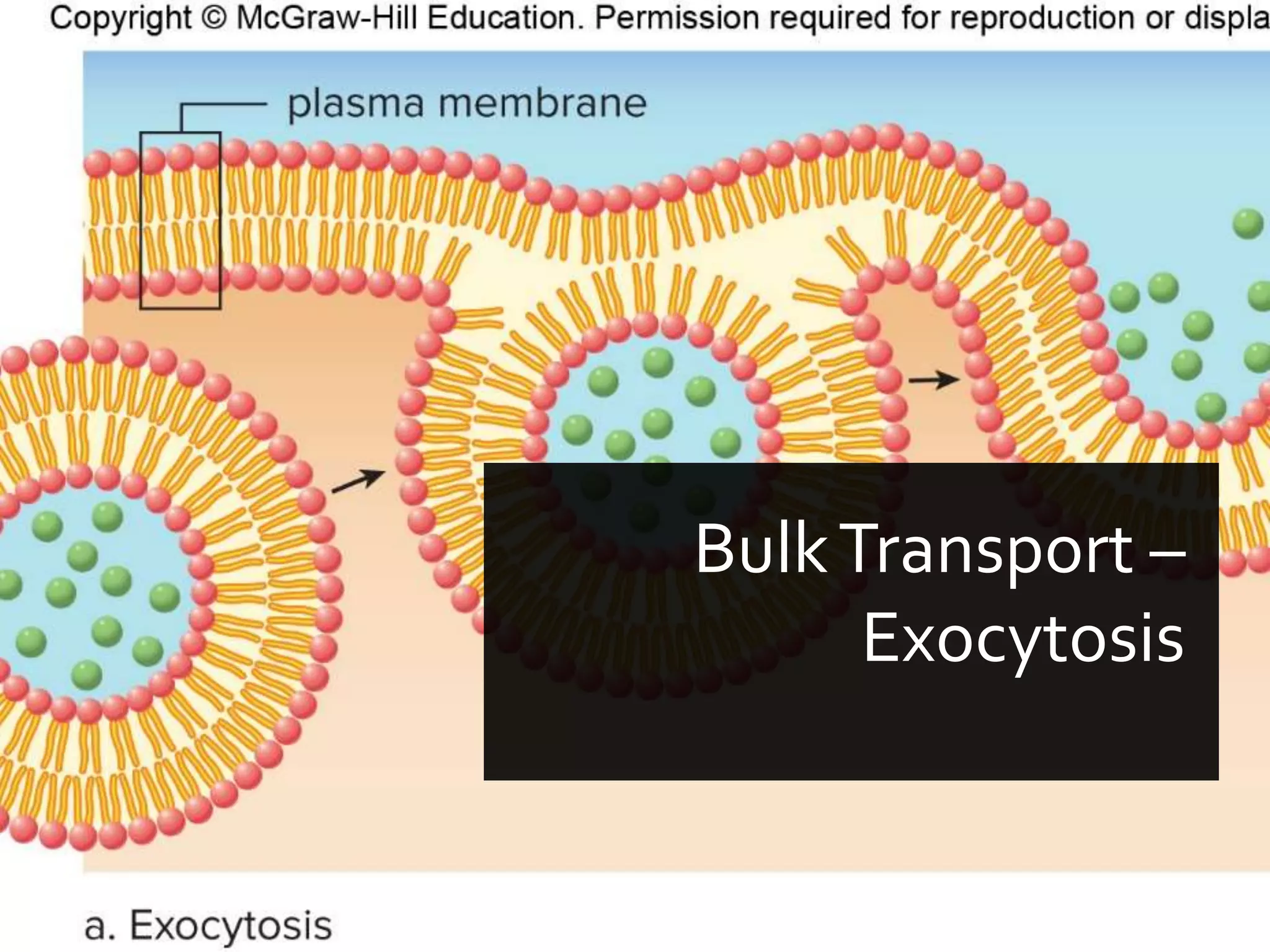
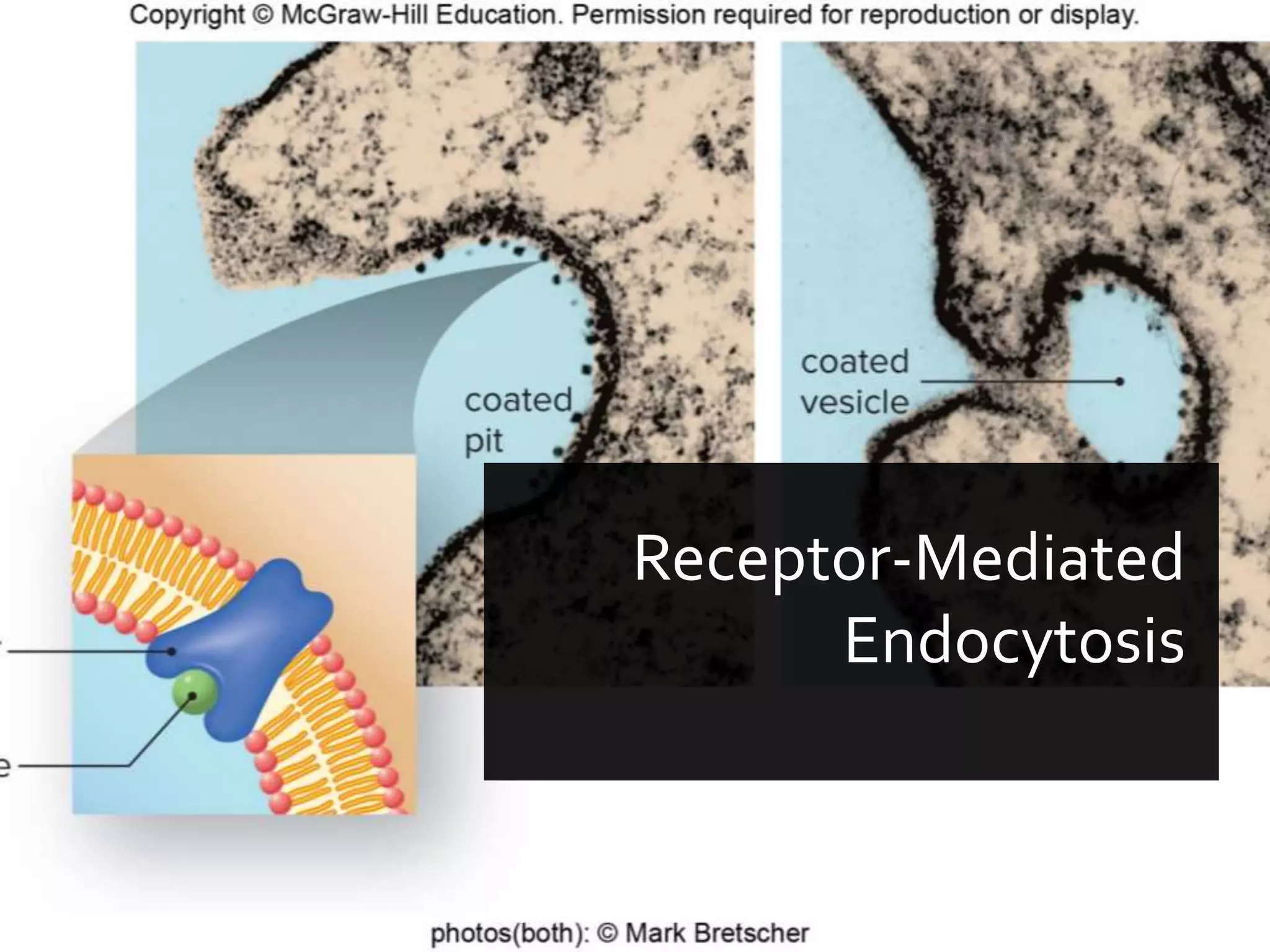
- The document discusses energy, metabolic pathways, enzymes, and their roles in cells. It covers the following key points:
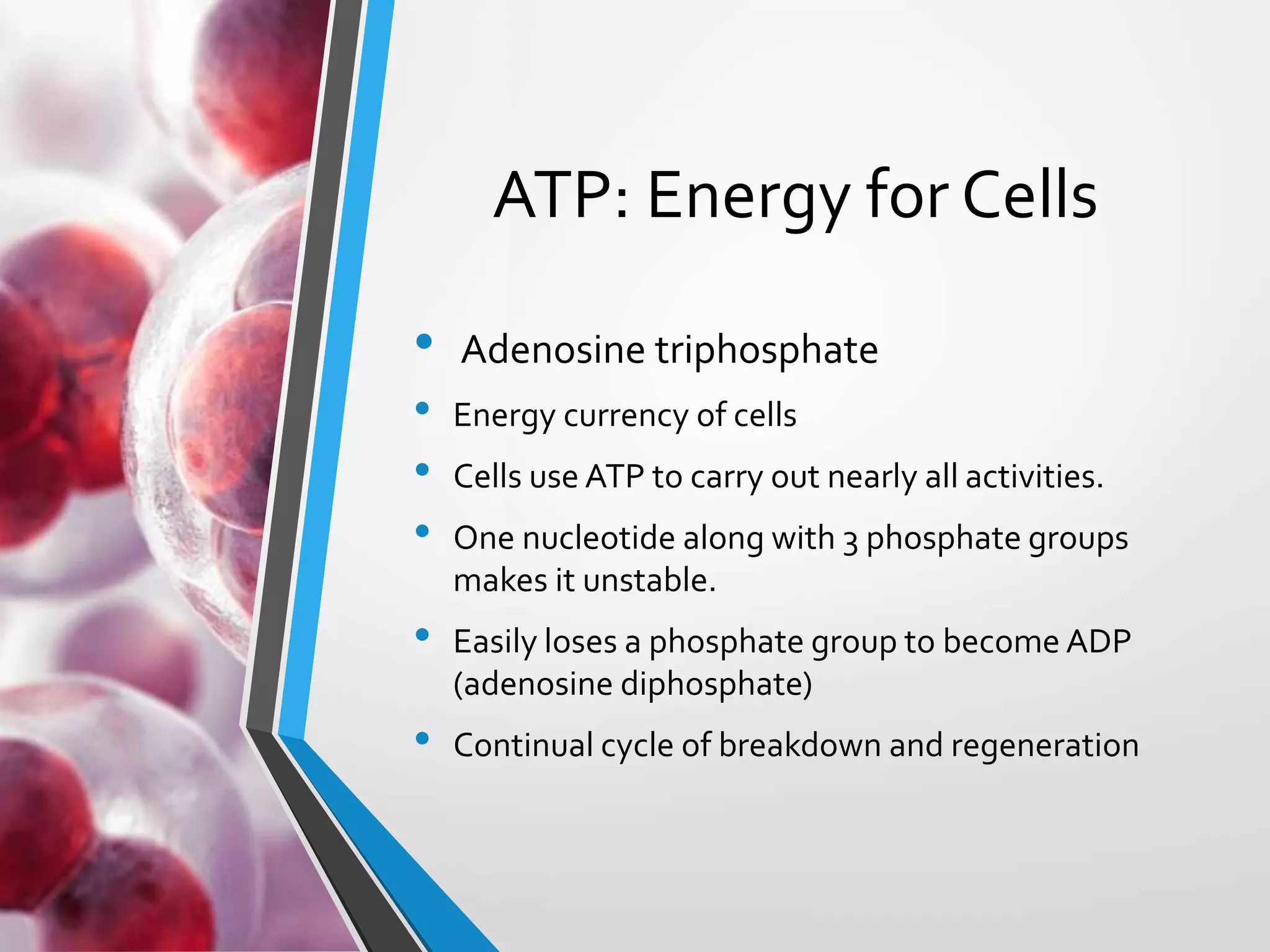
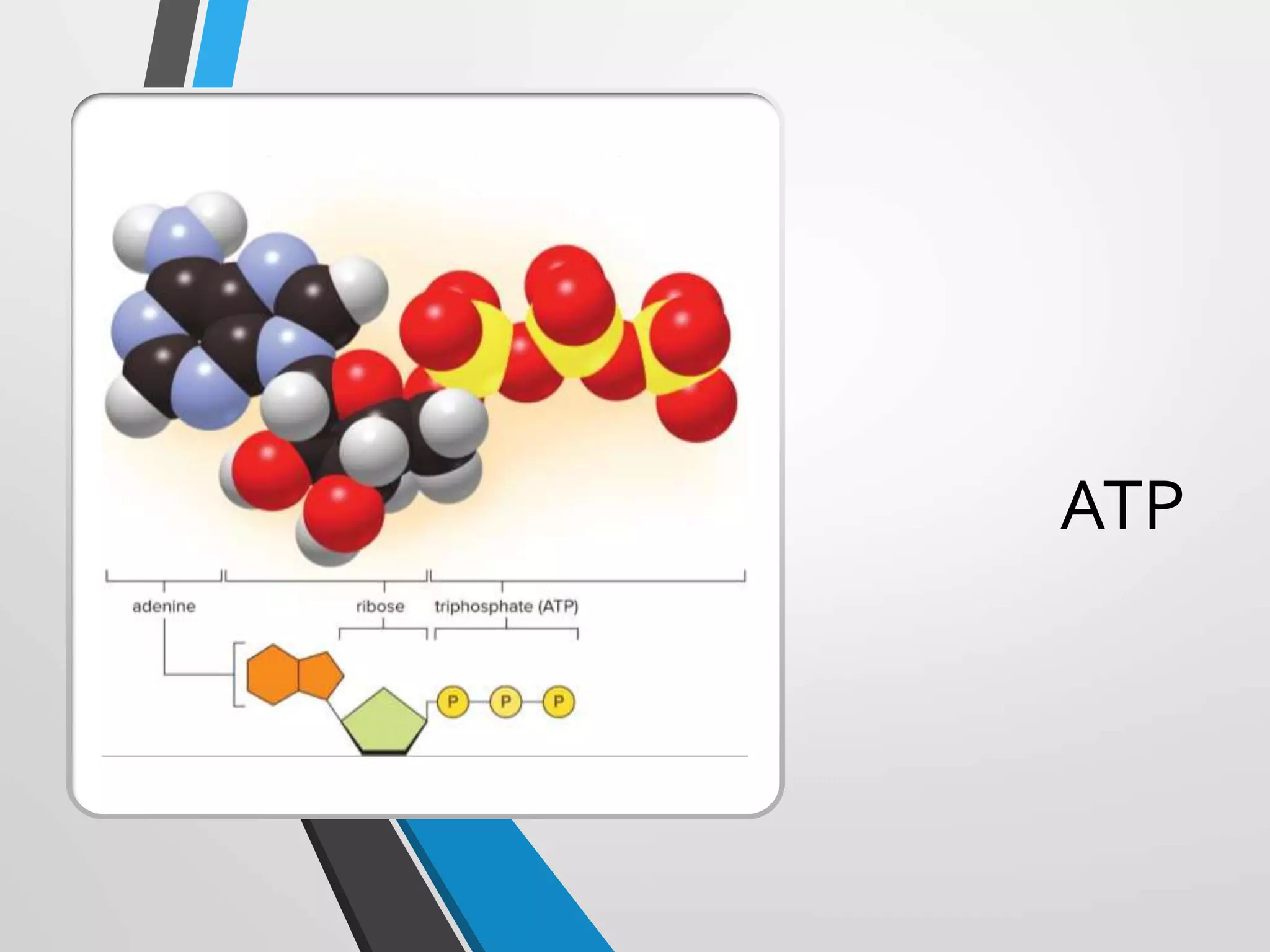
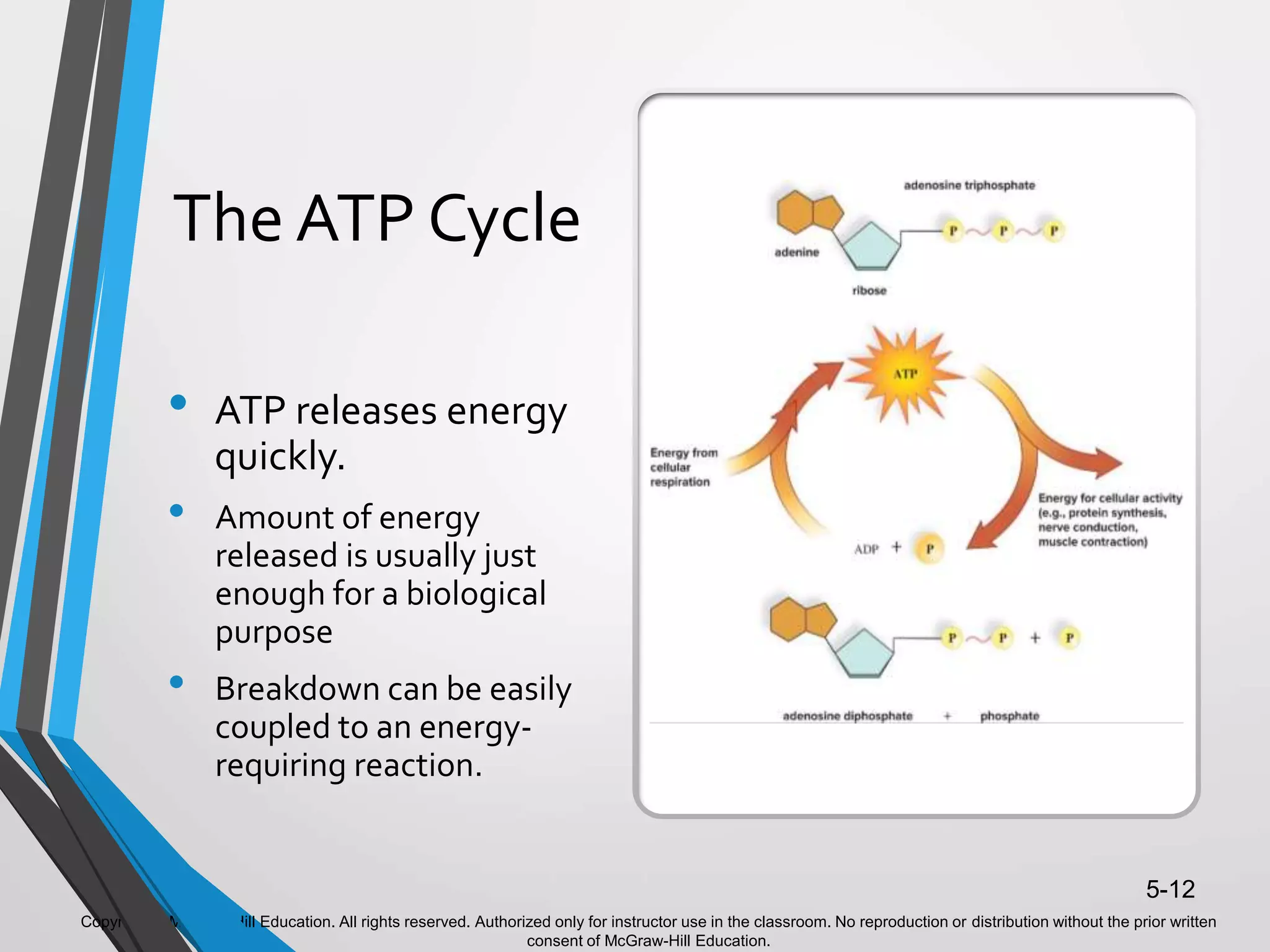
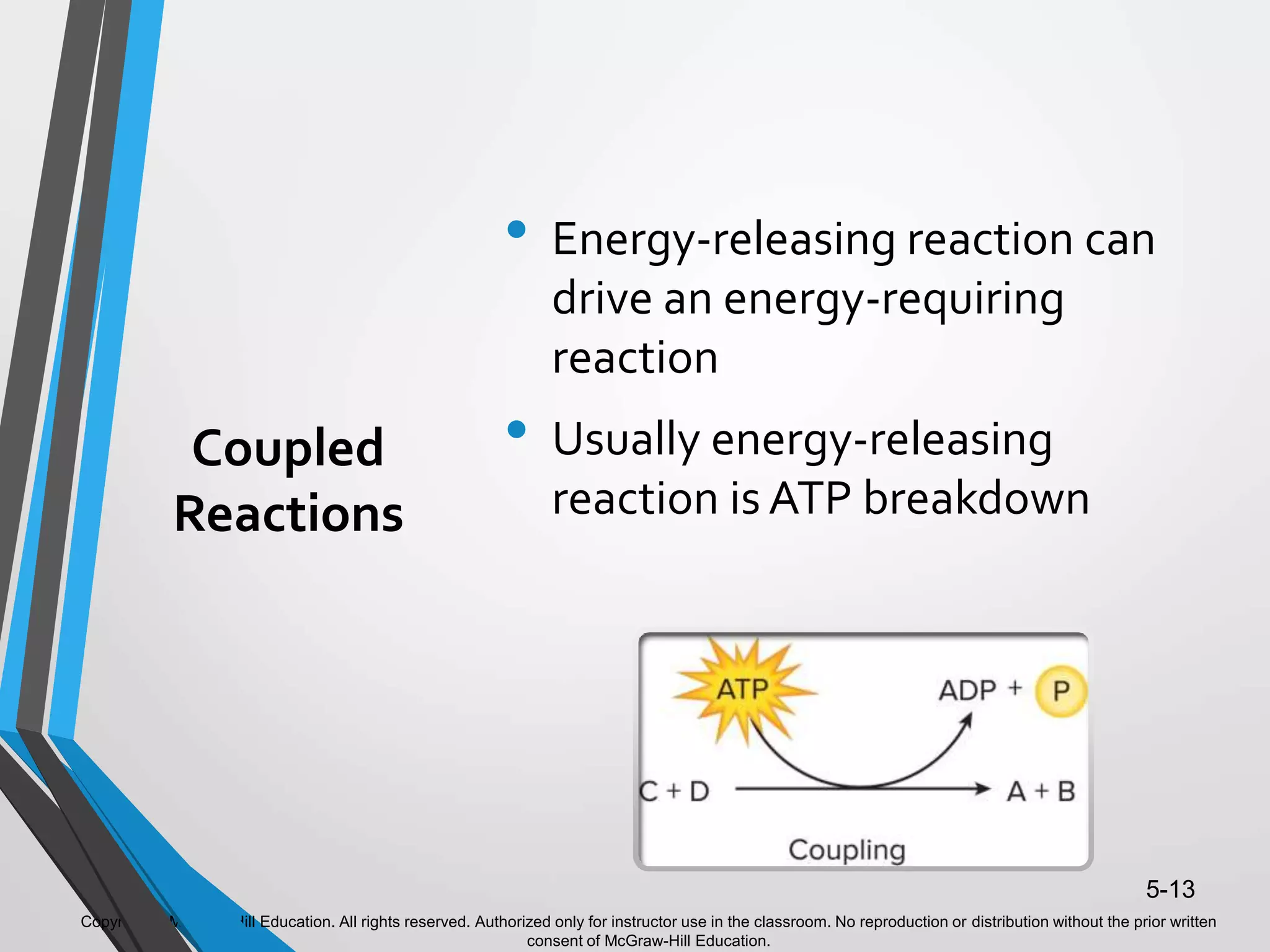
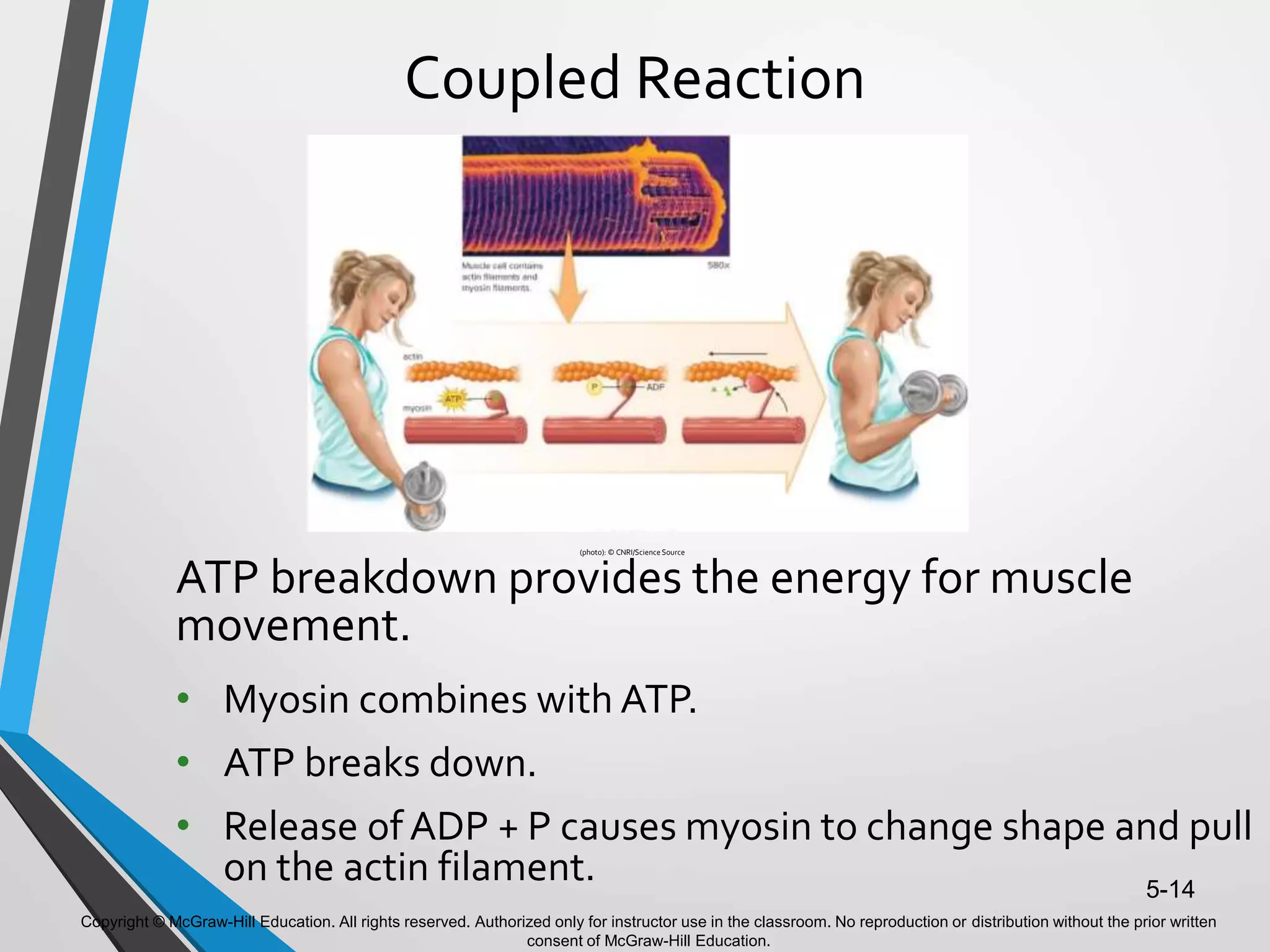
- ATP acts as the "energy currency" of cells, storing and transporting chemical energy within cells to power metabolic reactions. ATP is regenerated through coupled reactions with energy-releasing steps like ATP breakdown.
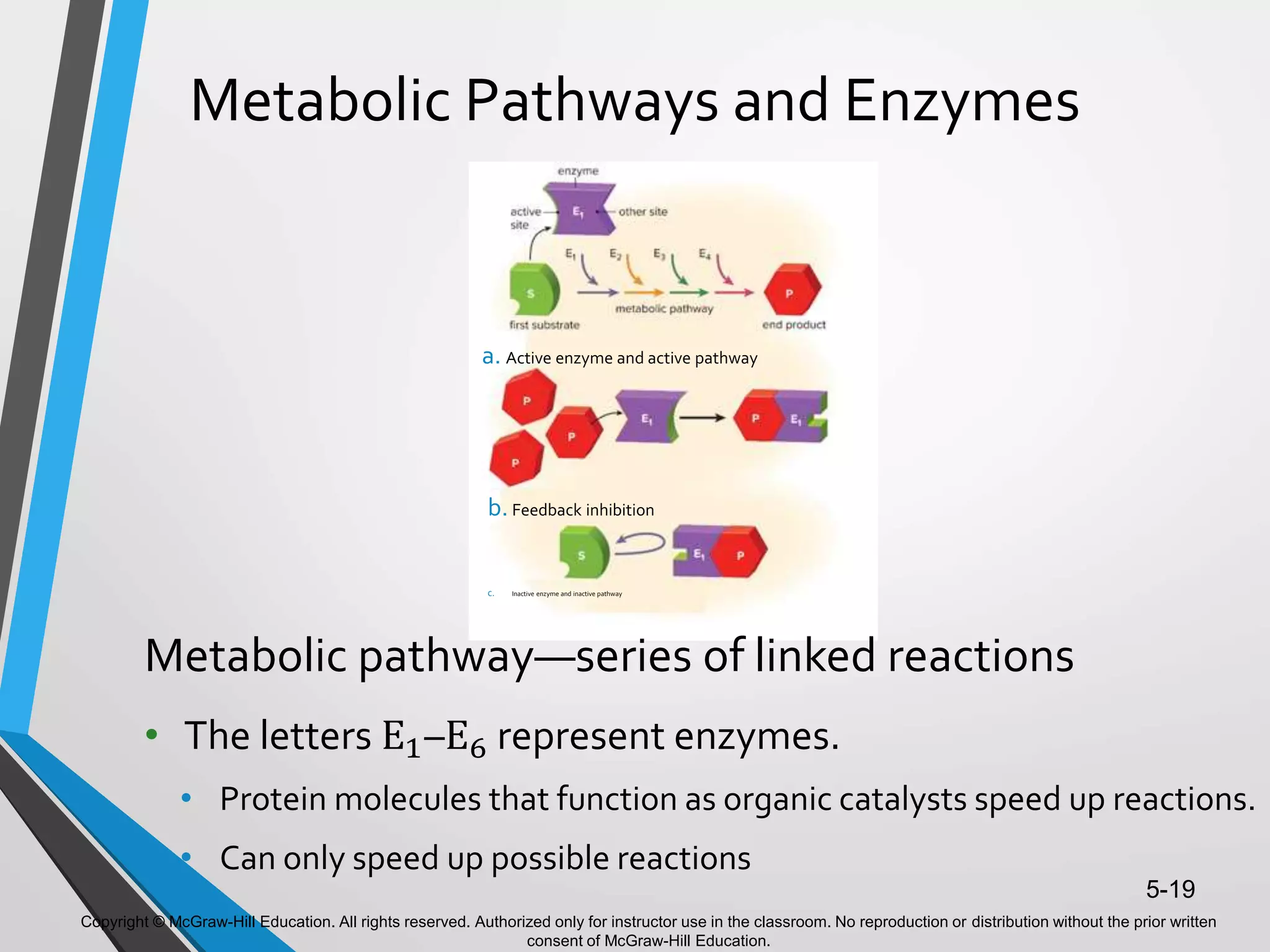
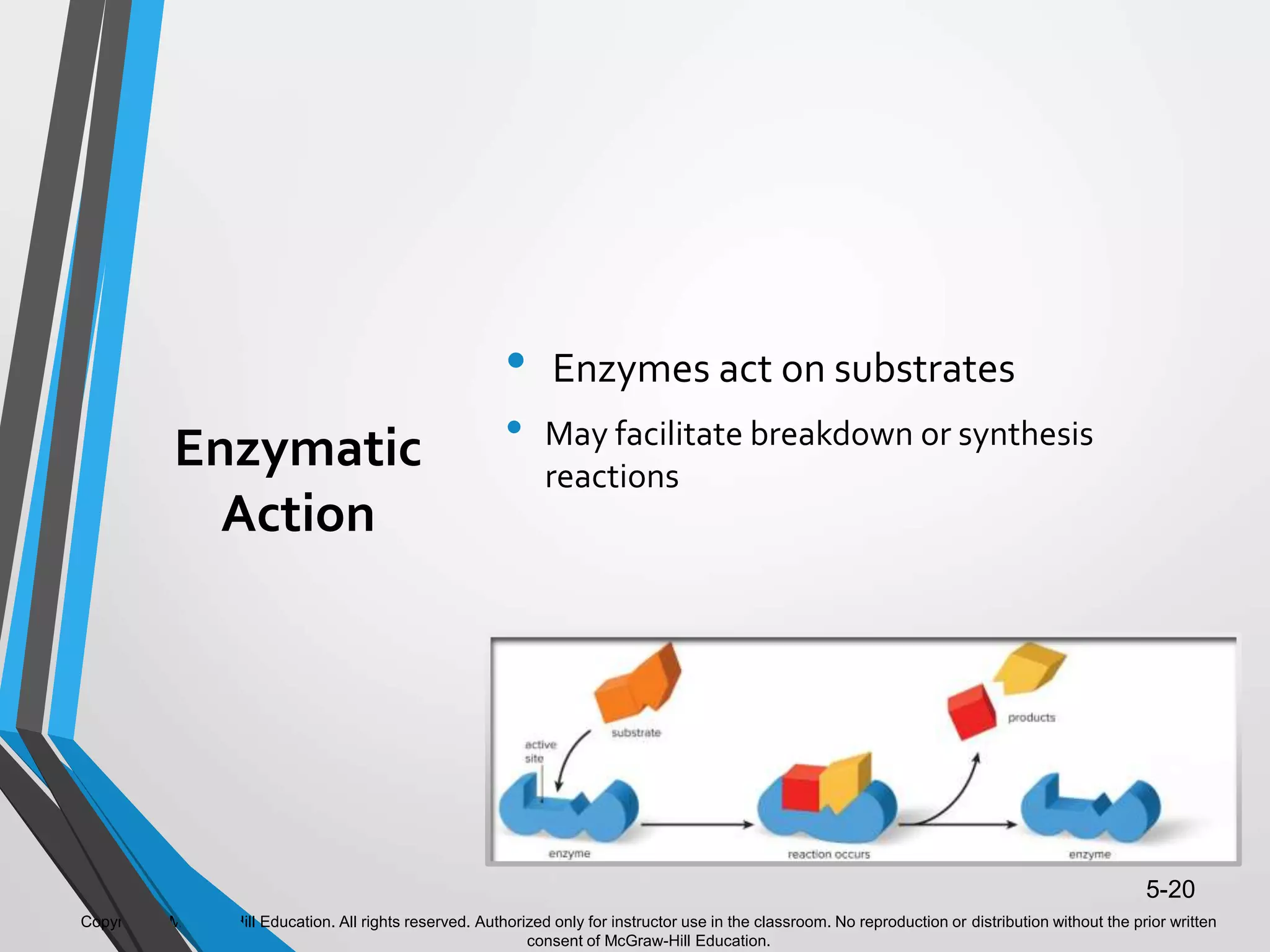
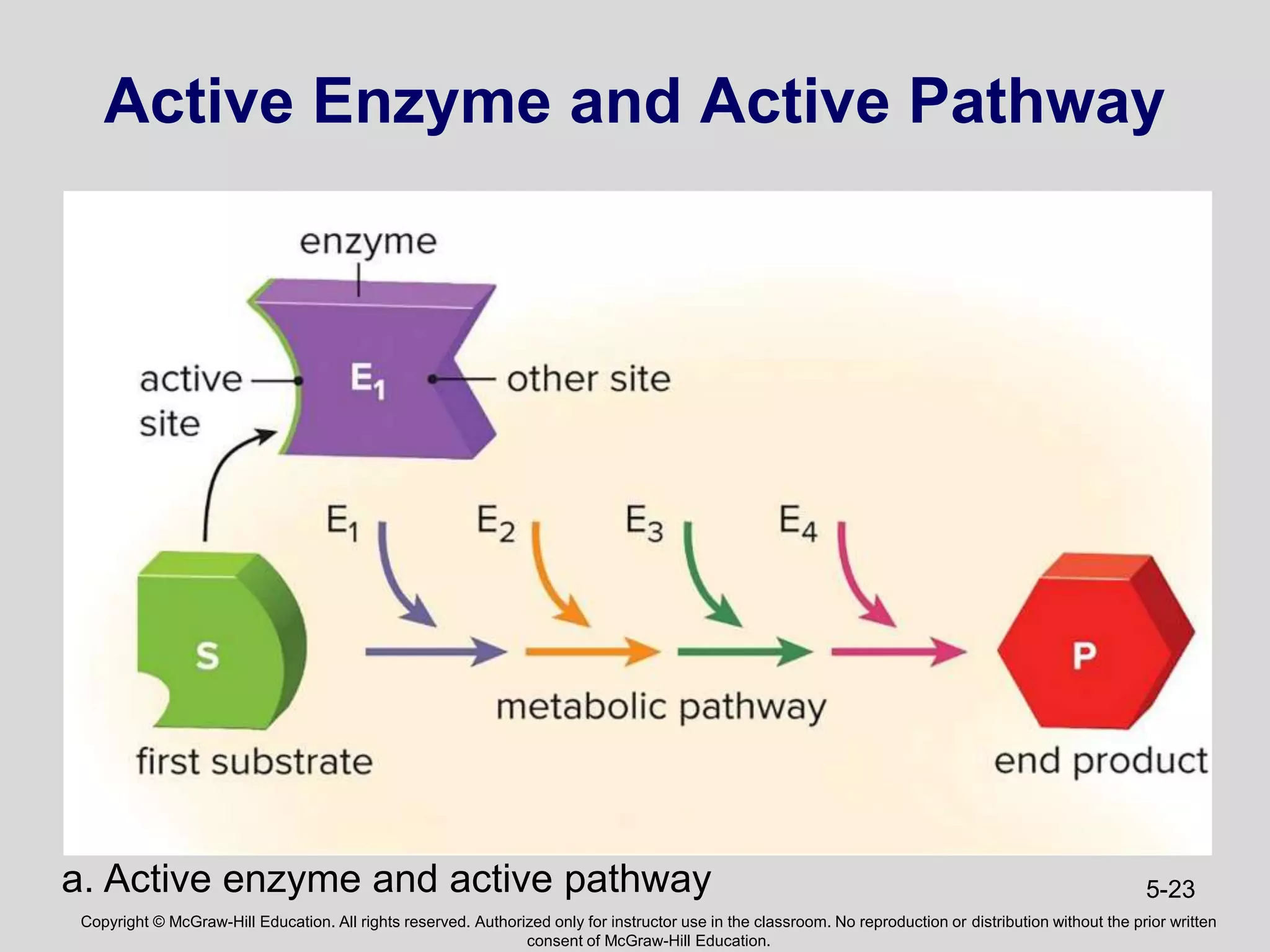
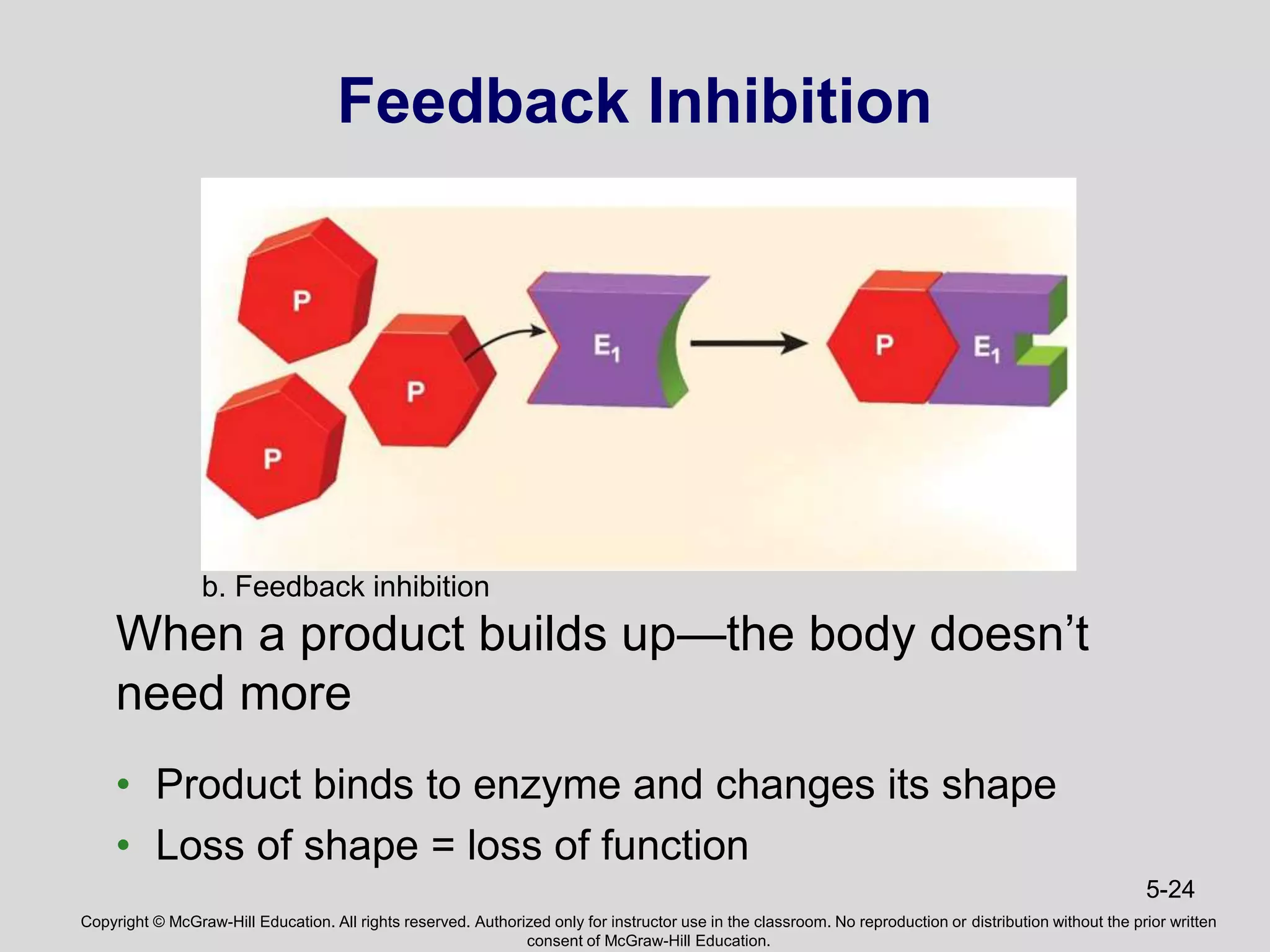
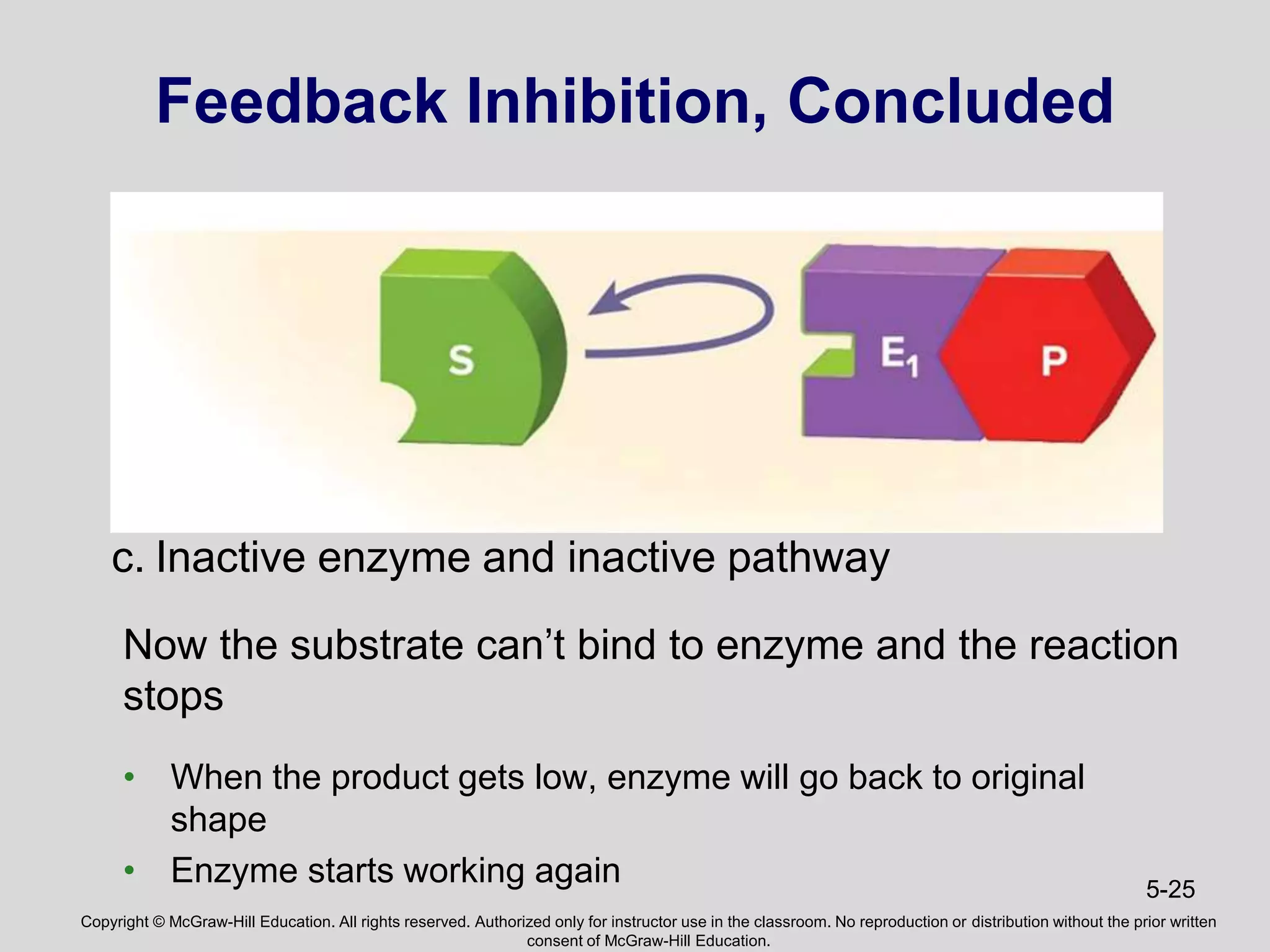
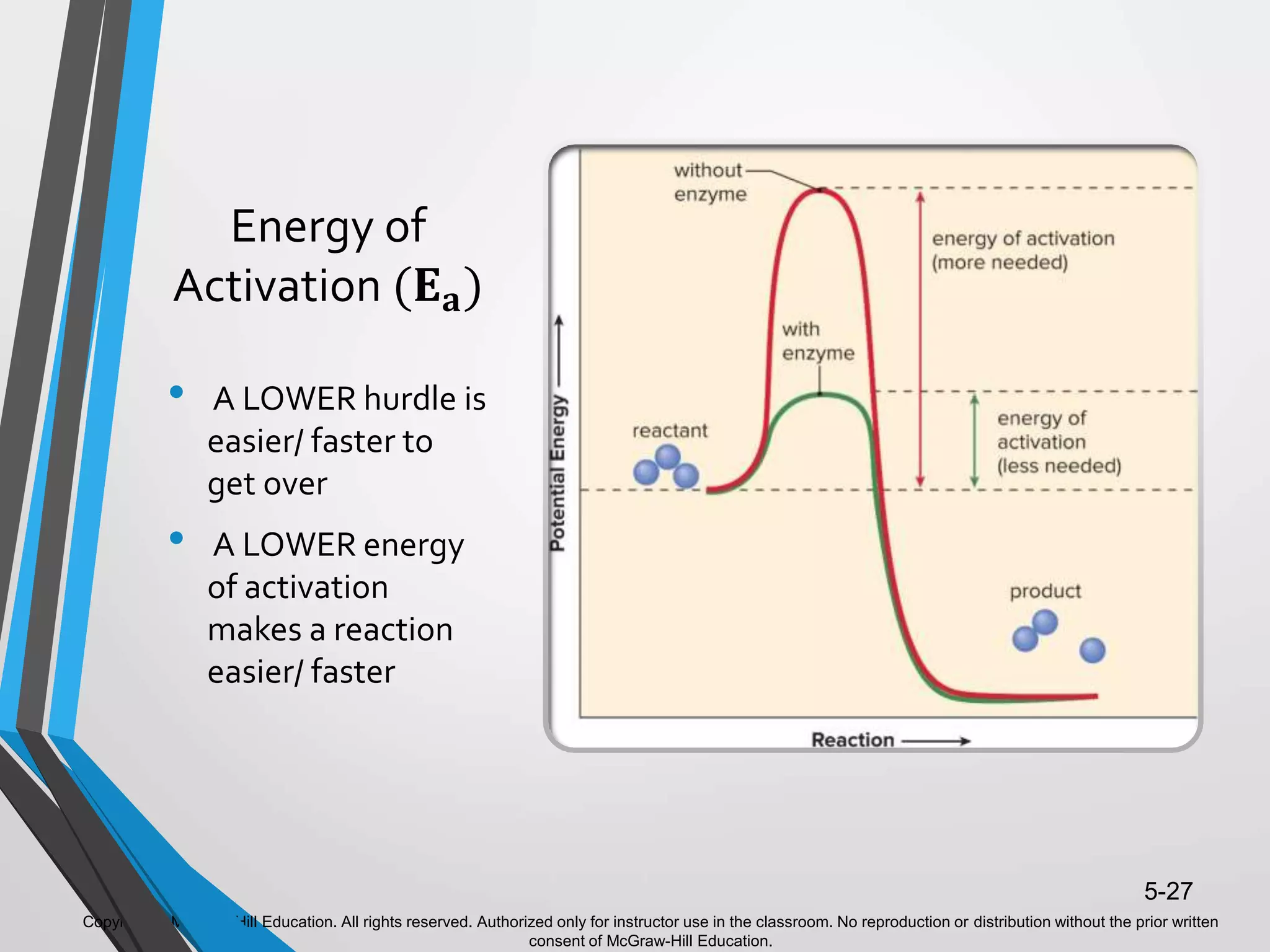
- Enzymes catalyze metabolic pathways, speeding up biochemical reactions without being used up. They have specific active sites that facilitate reactions on substrates. Feedback inhibition regulates pathways by shutting them down when products accumulate.
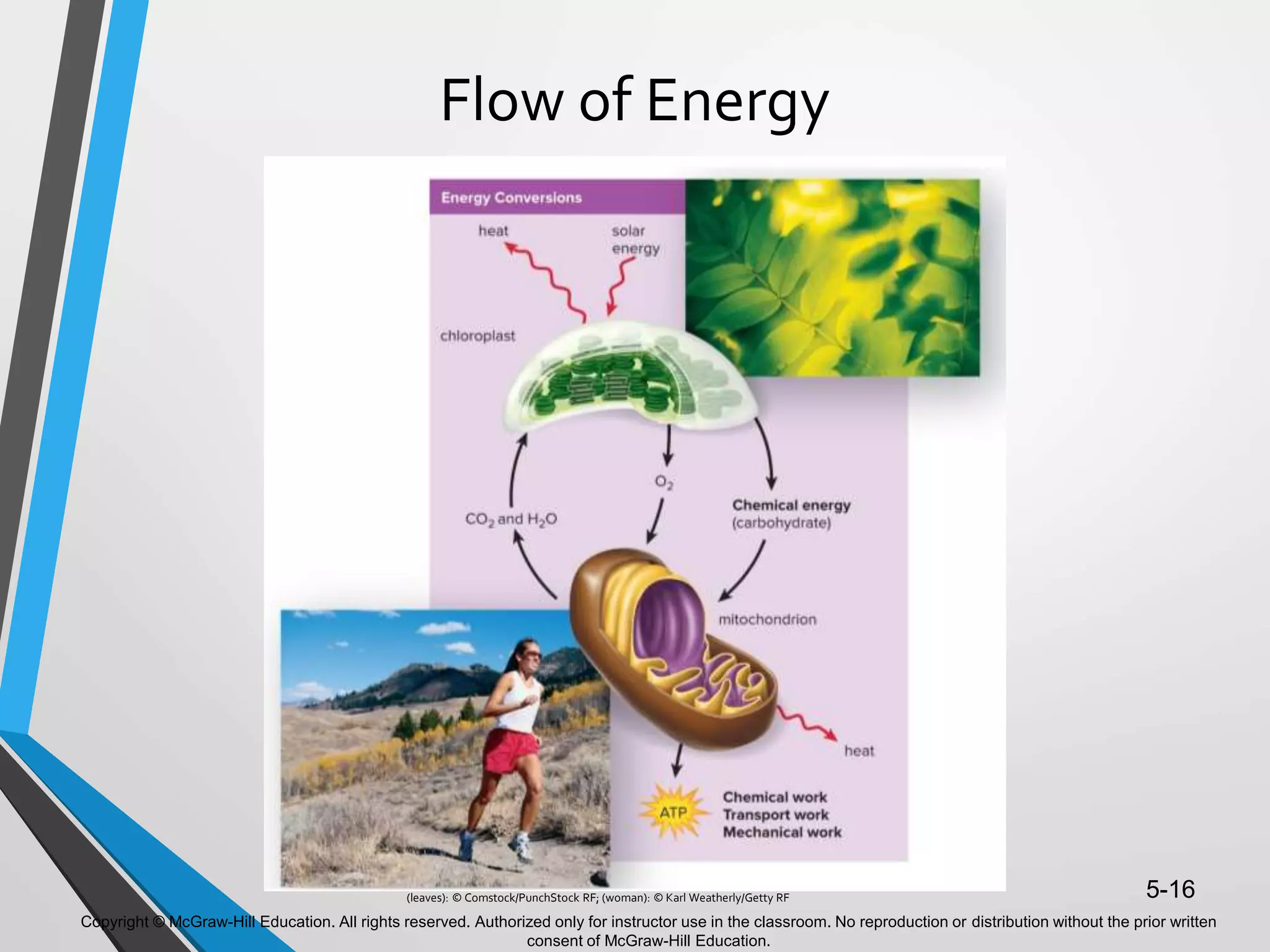
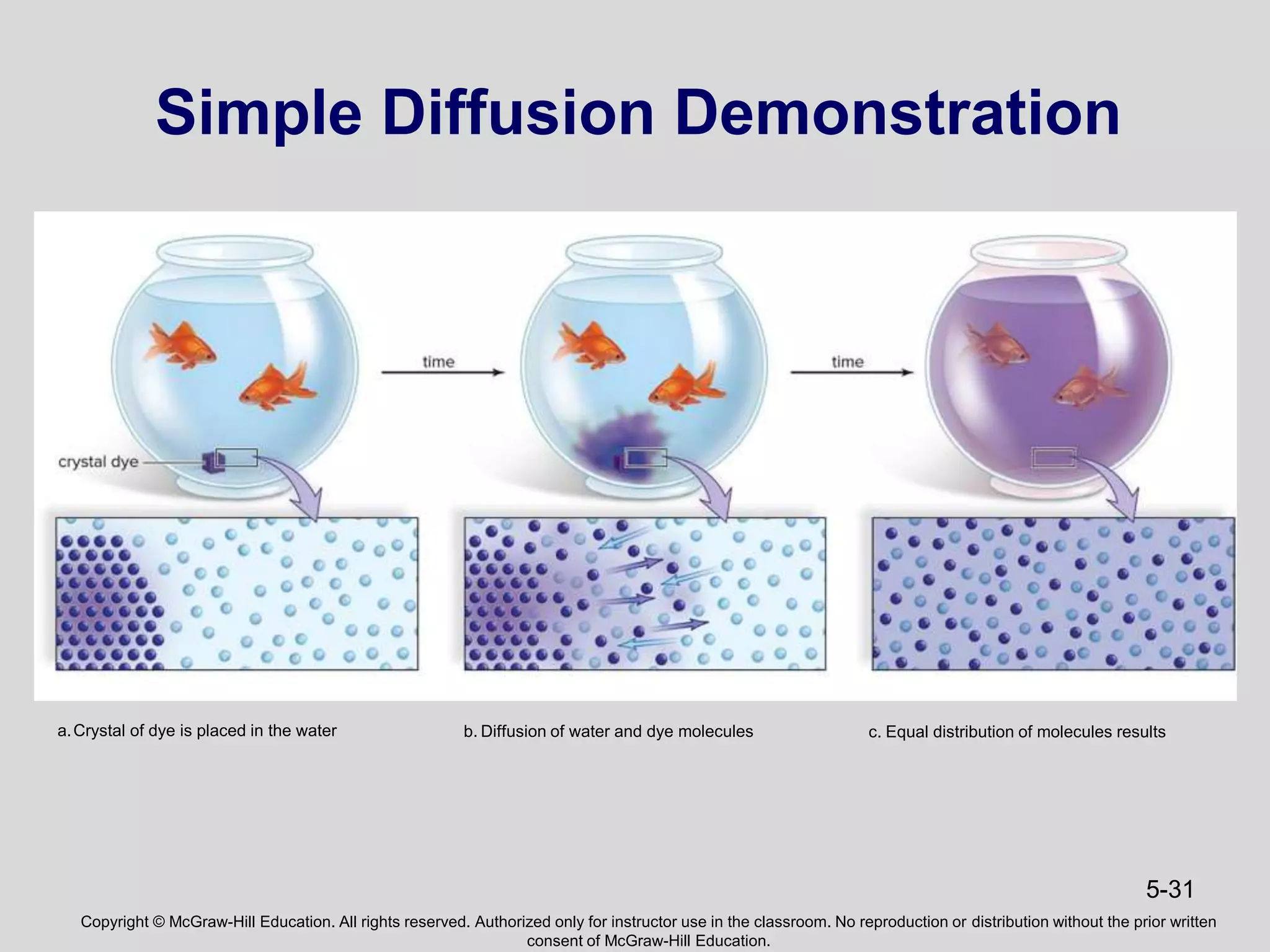
- Energy from the sun powers photosynthesis, producing carbohydrates that are broken down through cellular respiration to regenerate ATP. This cycling of energy between organisms maintains the flow