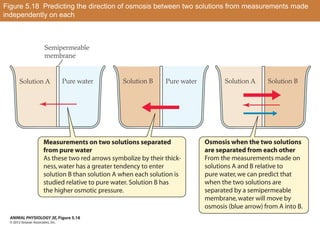
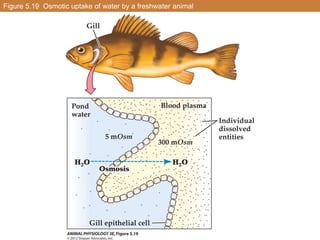
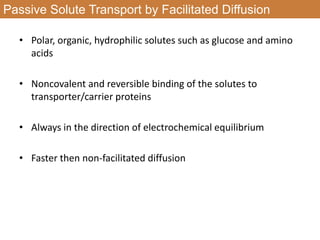
This document summarizes different types of transport mechanisms in animal cells. It describes passive transport mechanisms like facilitated diffusion that move solutes down their electrochemical gradient. It also describes active transport mechanisms that use energy (ATP) to move solutes against their gradient, including primary active transport via ATPases and secondary active transport using solute gradients. It discusses the coupling of solute transport and defines terms like cotransport and countertransport. Finally, it covers colligative properties of solutions like osmotic pressure and freezing point depression that depend on the number of dissolved particles.


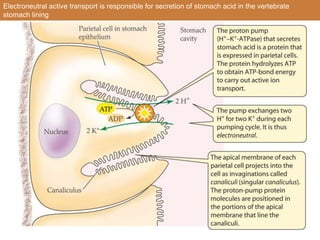

![Secondary Active Transport
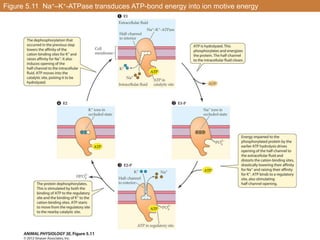
• Draws energy from an electrochemical gradient of a solute
• Usual mechanism of transport for organic solutes
• ATP is used to create the gradient
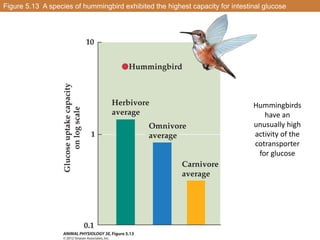
• Example: glucose absorption in the small intestine of the
hummingbird
-glucose is moved from [low][high]
-it is carrier-mediated
-the energy source for the uphill transport is metabolism:
draws energy from an electrochemical gradient of a
solute](https://image.slidesharecdn.com/animalphyschapter5part2-151006190724-lva1-app6891/85/Animal-phys-chapter-5-part-2-8-320.jpg)






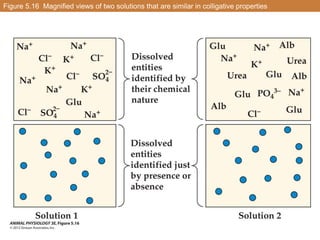
![Raising the concentration of dissolved entities in a solution increases the
osmotic pressure of the solution and lowers the solution’s freezing point and
water vapor pressure the osmotic pressure is proportional to the
concentration of dissolved entities
For example: doubling the concentration of solutes doubles the osmotic
pressure
However, doubling [solute] doubles the difference between the freezing point
or water vapor pressure of a solution and that of pure water (freezing-point
or water-vapor depression)](https://image.slidesharecdn.com/animalphyschapter5part2-151006190724-lva1-app6891/85/Animal-phys-chapter-5-part-2-18-320.jpg)