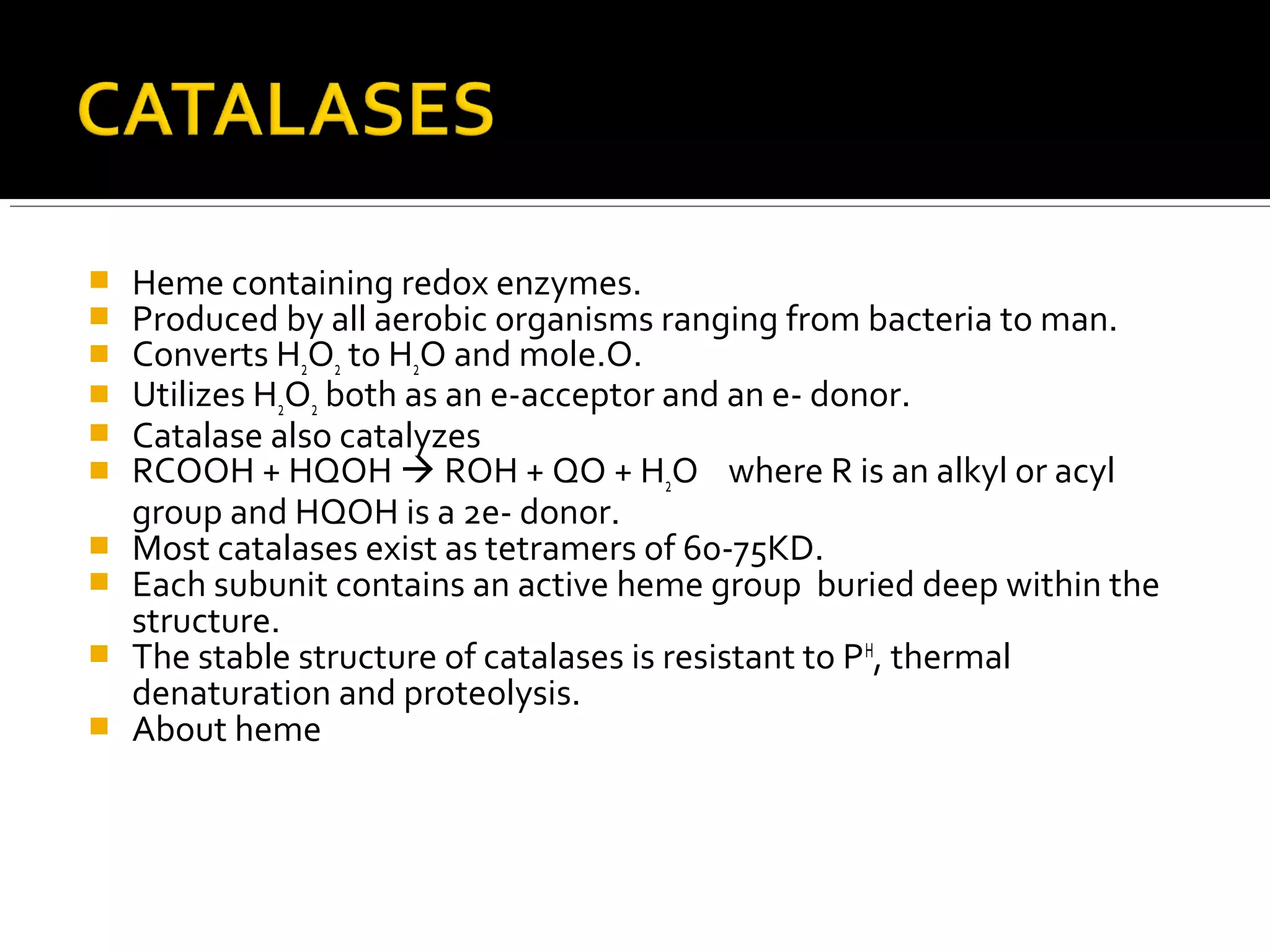
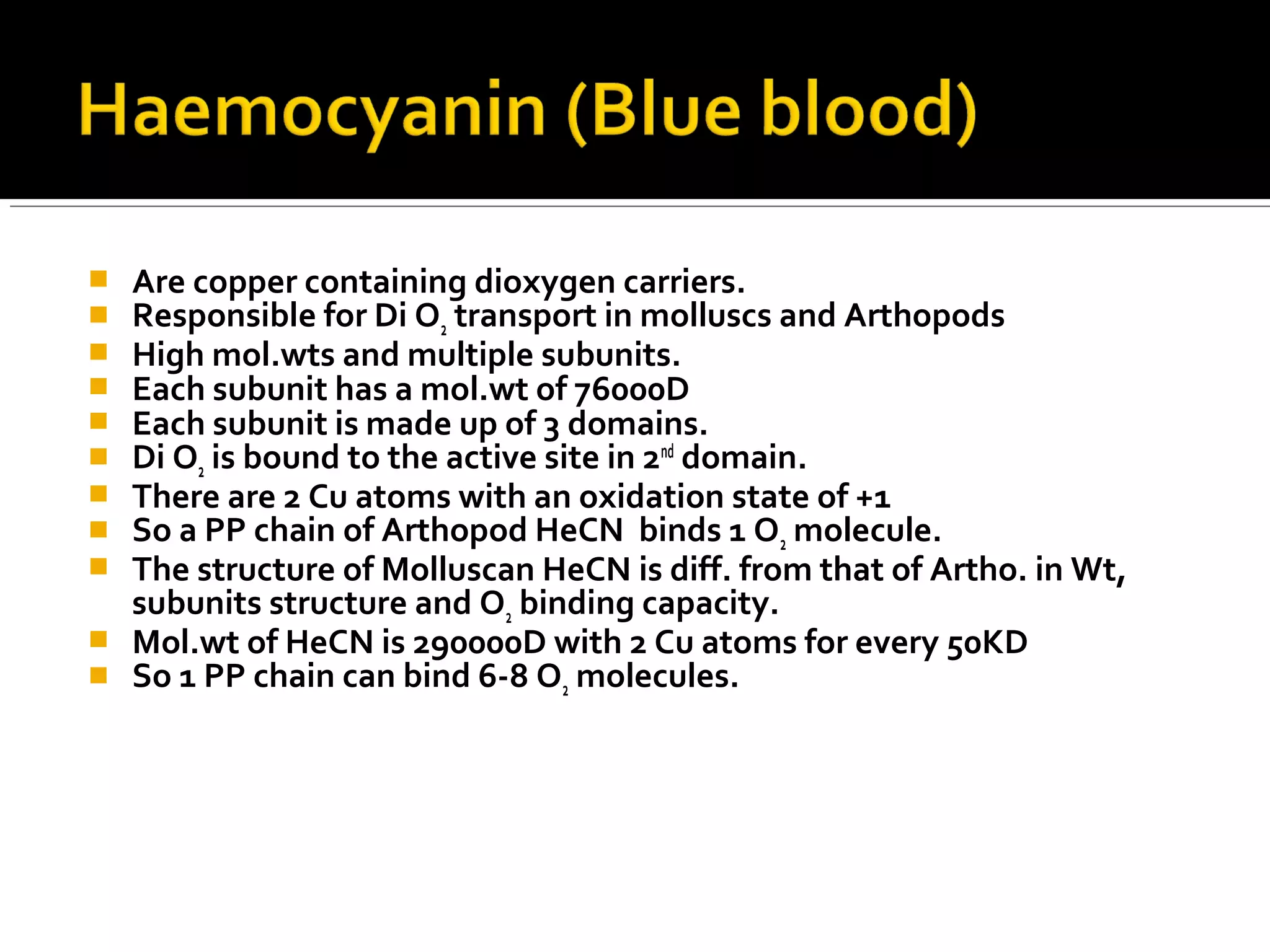
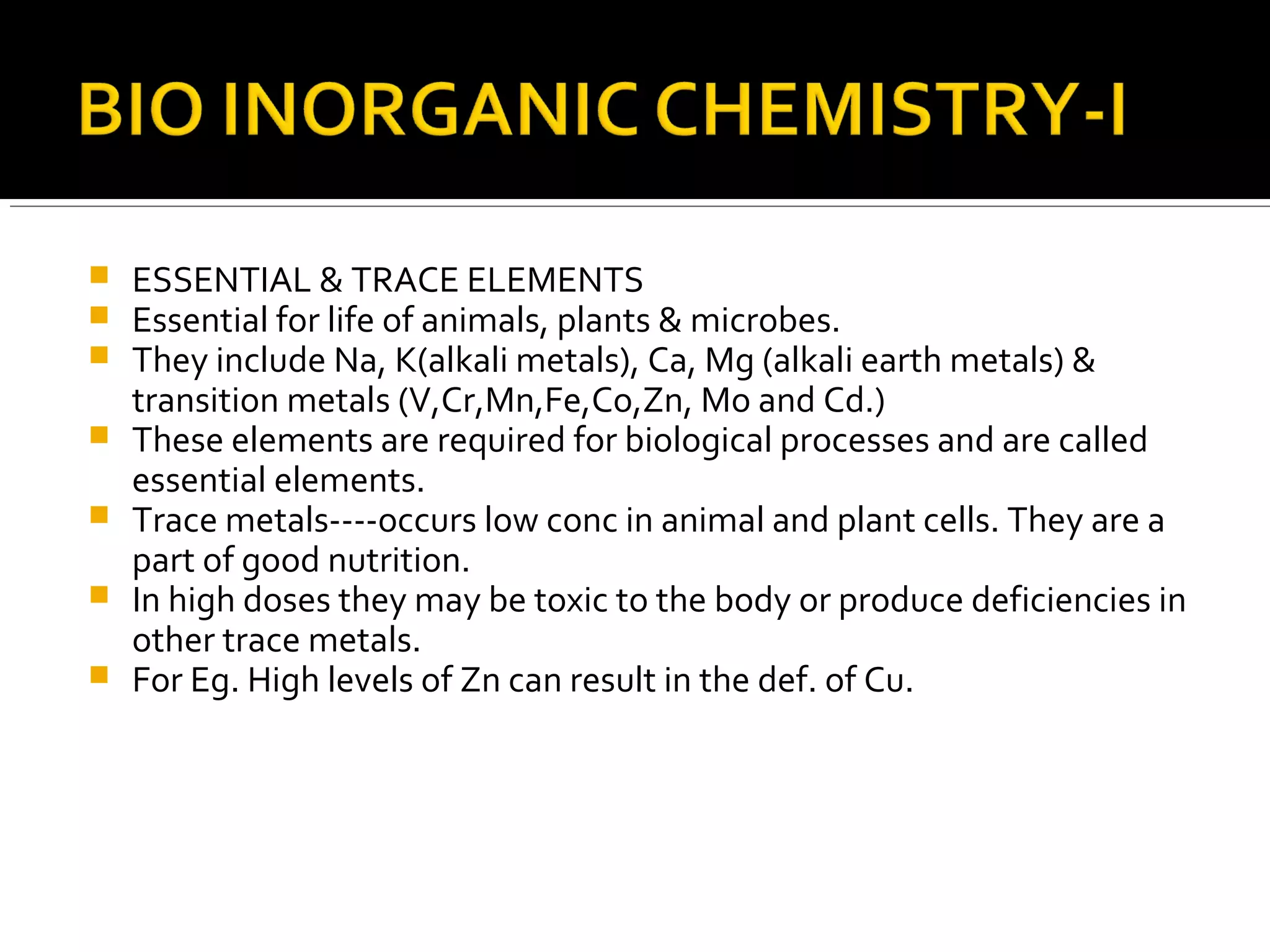
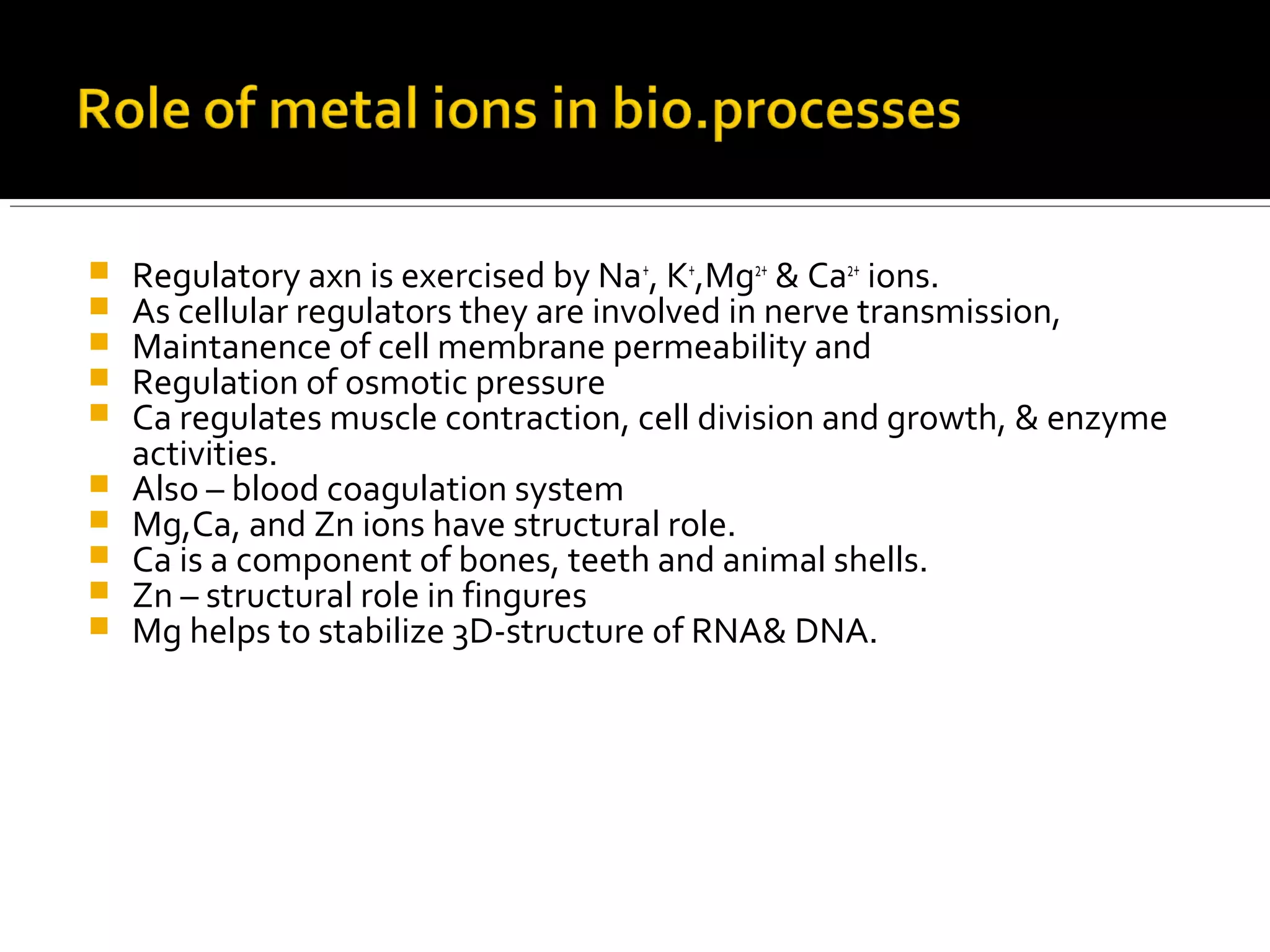
This document discusses the electron transport chain (ETC) and its components. It notes that the ETC is located in the inner mitochondrial membrane and utilizes electrons derived from nutrients to generate ATP through a series of oxidation-reduction reactions. It describes the five complexes of the ETC (Complexes I-IV which transport electrons and Complex V which synthesizes ATP) as well as the mobile carriers involved in electron transport, including NADH, Coenzyme Q, cytochrome c, and oxygen. The ETC functions to transfer electrons from substrates to oxygen and harness the energy to produce ATP, making mitochondria the powerhouse of the cell.