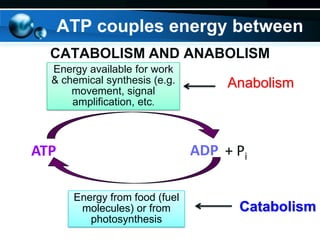
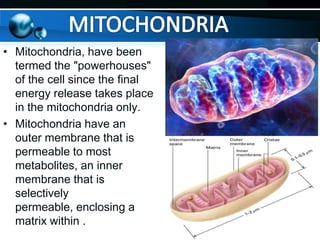
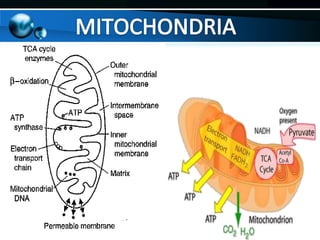
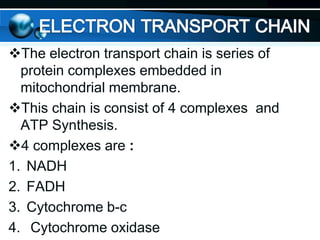
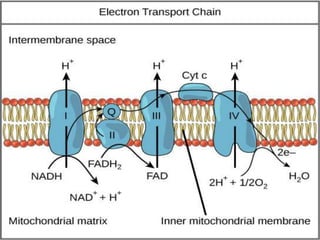
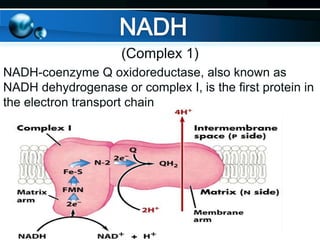
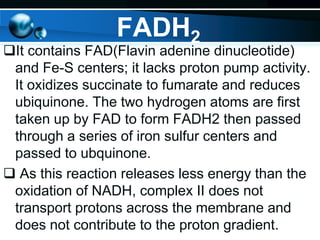
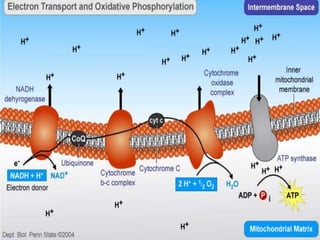
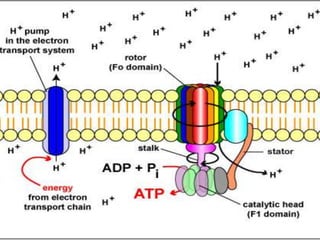
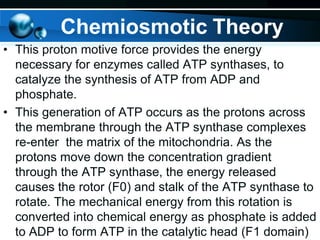
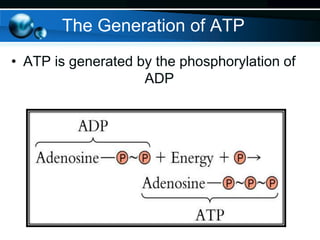
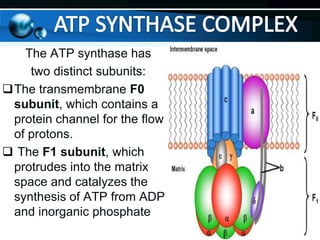
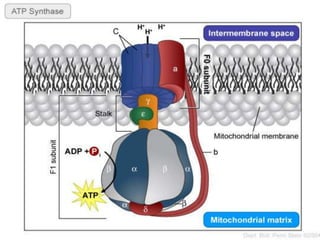
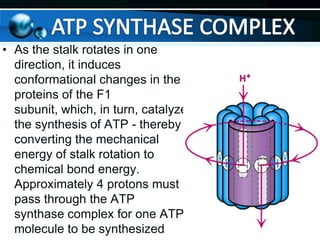
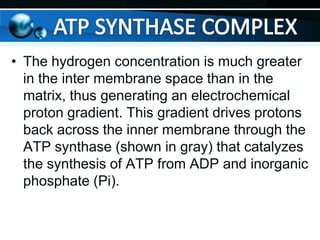
The document provides information on cellular respiration and how it generates ATP through oxidative phosphorylation in the mitochondria. It discusses the electron transport chain, made up of protein complexes I-IV in the inner mitochondrial membrane, which establishes a proton gradient by pumping protons from the matrix to the intermembrane space. This proton gradient drives ATP synthase to catalyze the phosphorylation of ADP to ATP. The chemiosmotic theory explains how the potential energy in the proton gradient is used to produce ATP through rotation of the ATP synthase complex.