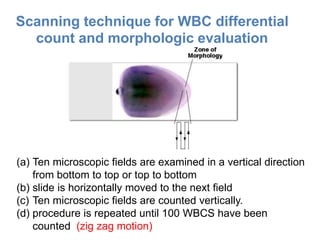
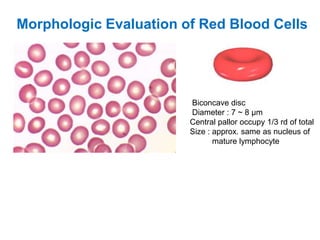
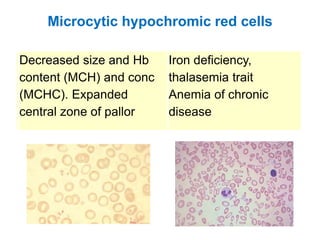
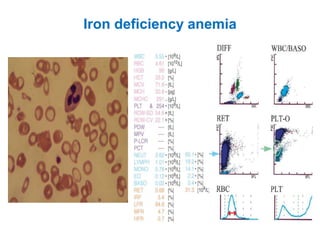
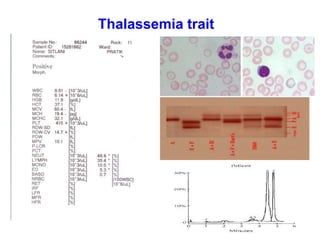
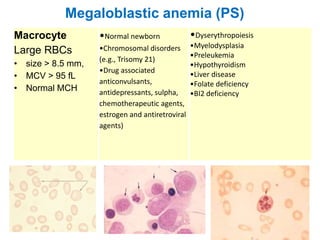
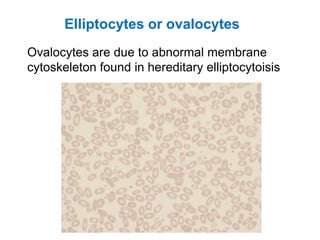
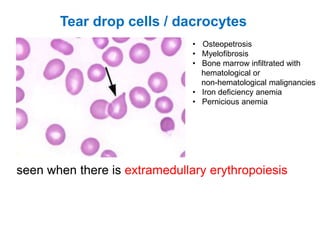
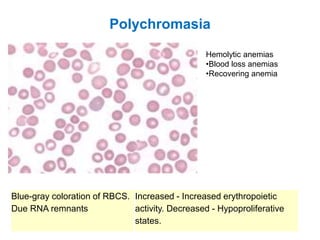
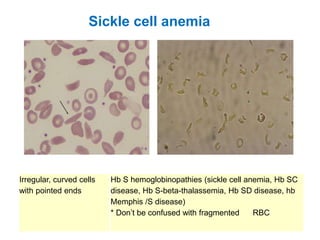
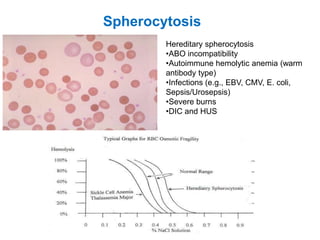
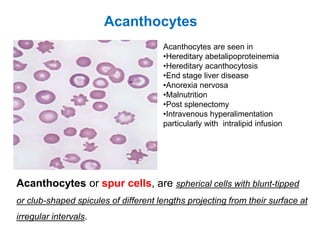
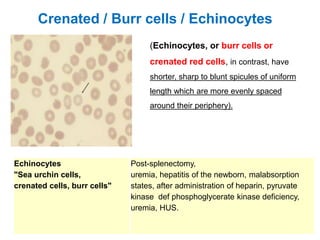
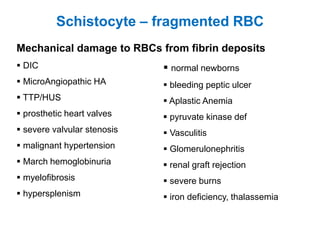
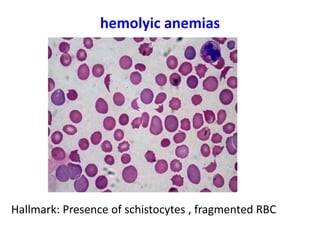
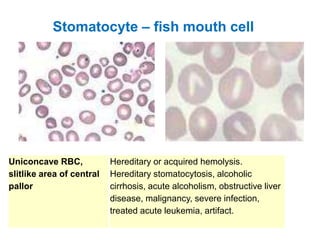
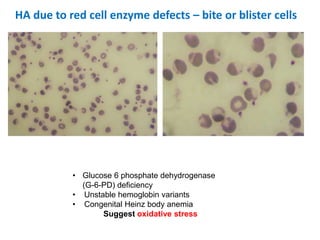
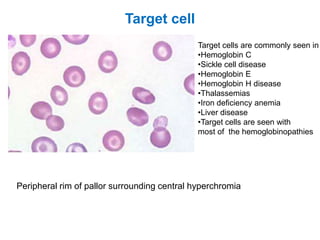
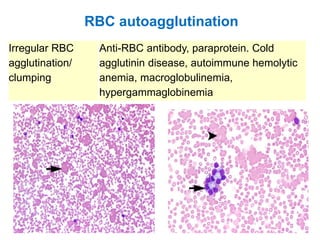
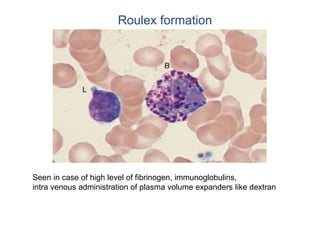
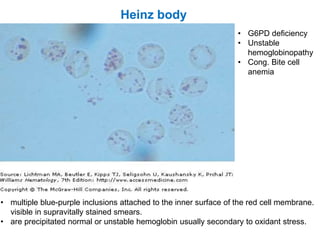
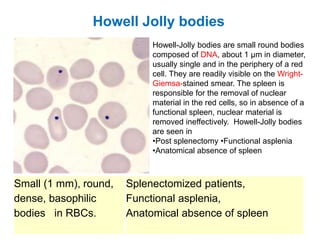
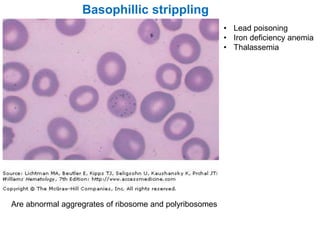
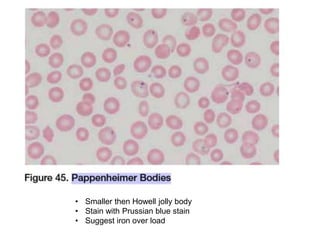
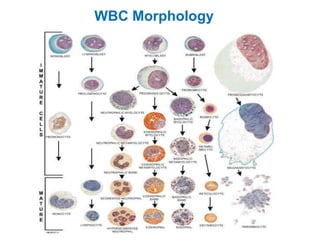
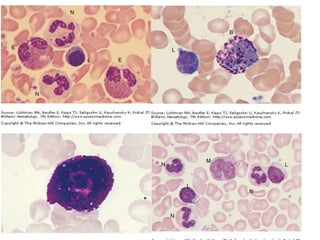
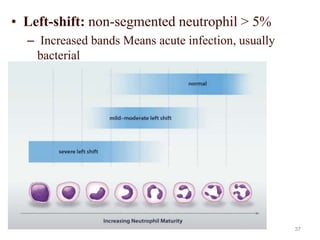
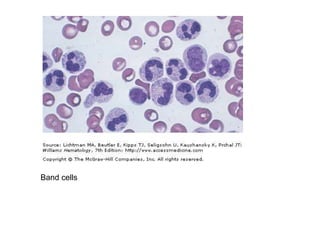
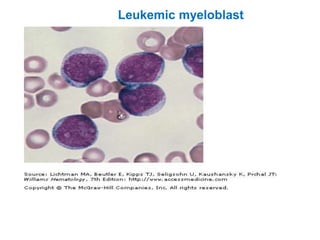
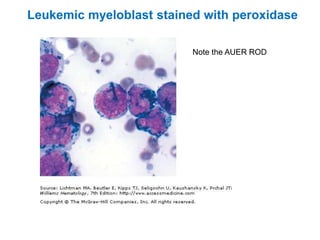
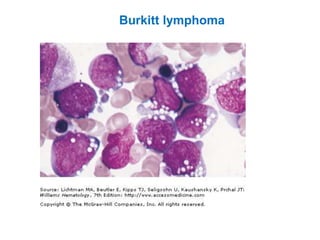
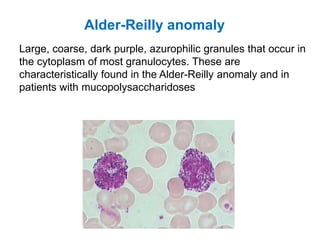
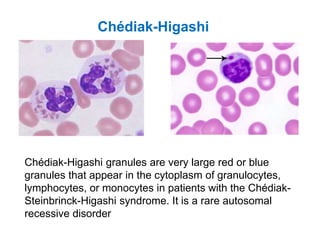
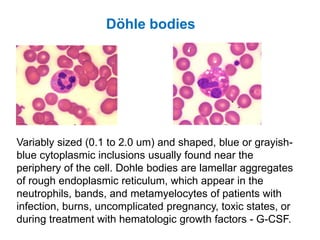
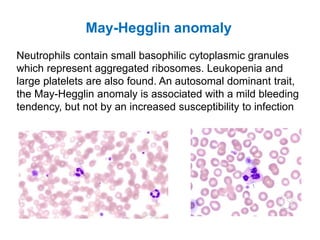
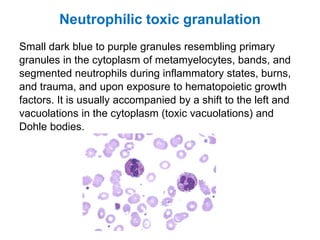
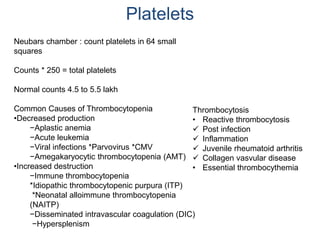
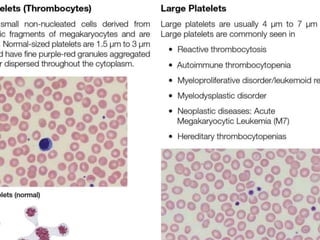
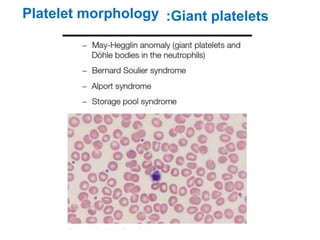
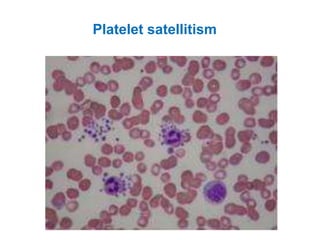
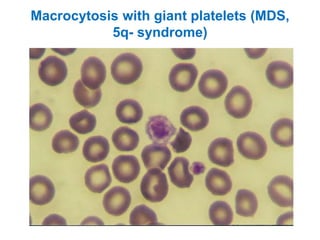
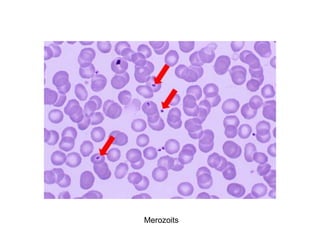
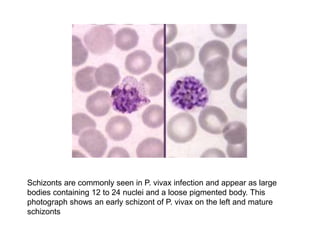
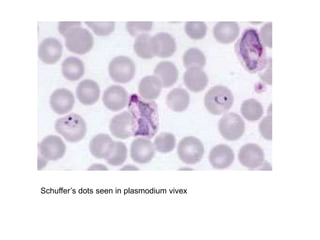
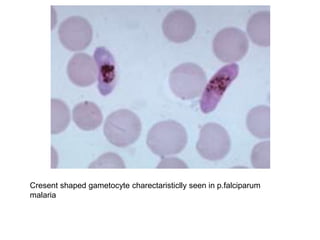
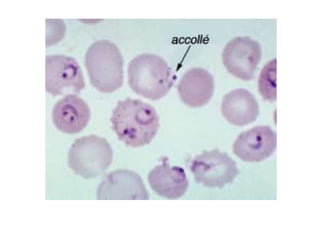
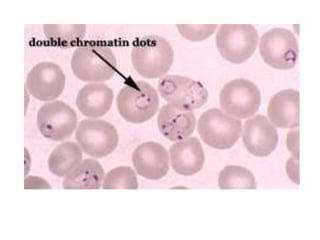
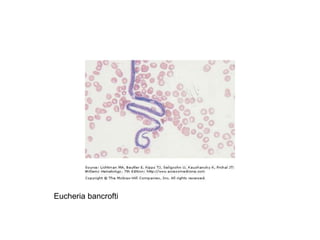
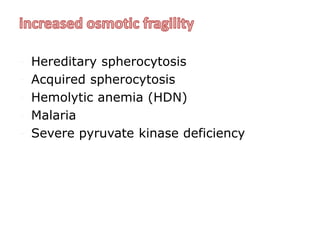
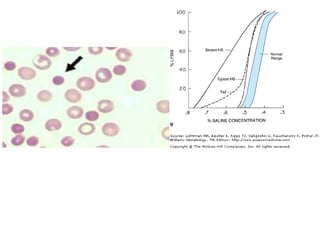
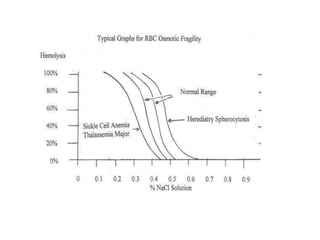
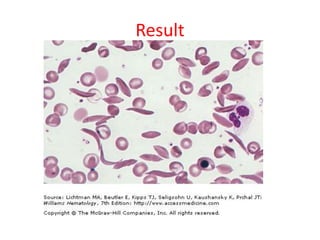
This document provides information about performing and interpreting a peripheral blood smear examination. It discusses preparing the smear, staining it using Romanowsky staining techniques, and systematically examining it under the microscope. The summary includes evaluating red blood cells for abnormalities in size, shape, inclusions and other features. White blood cell differential counts and platelet assessment are also reviewed. The document outlines various morphological abnormalities that may be observed and their potential clinical significance.