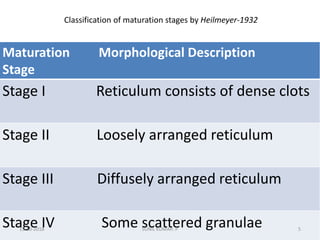
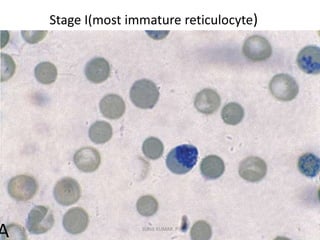
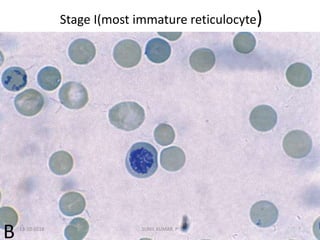
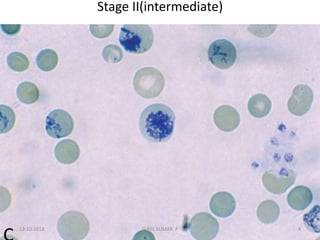
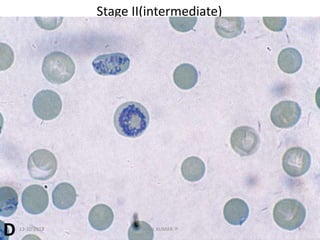
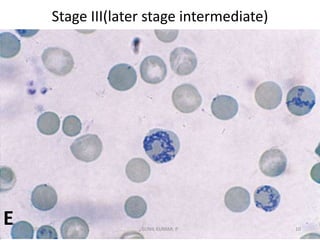
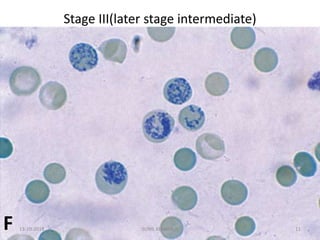
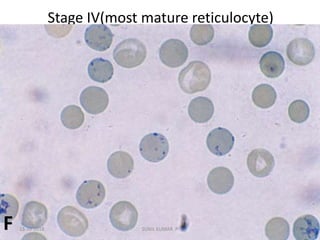
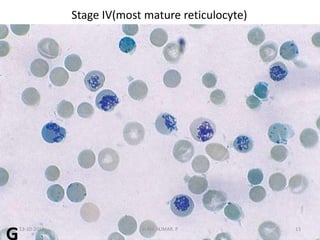
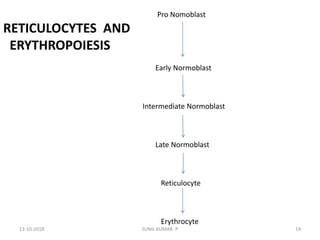
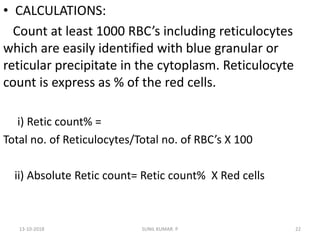
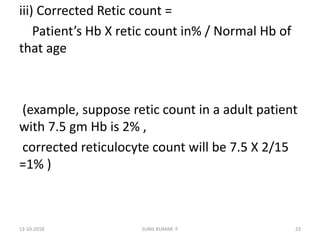
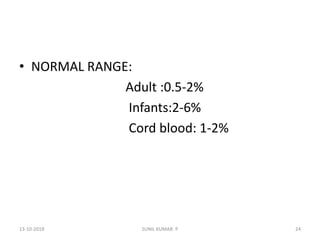
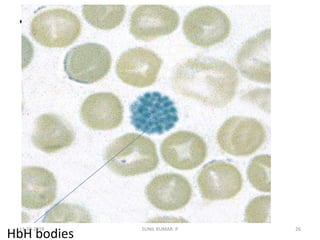
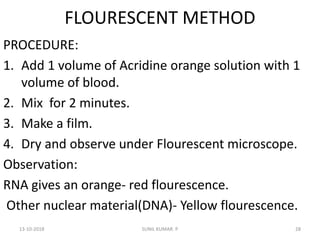
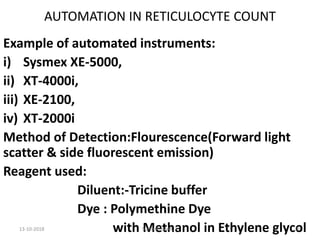
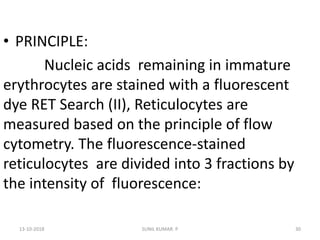
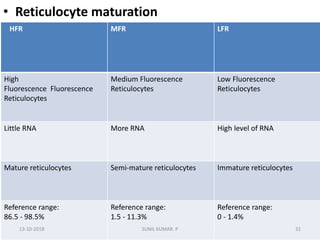
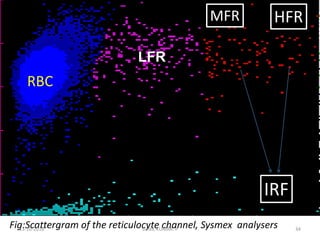
Reticulocytes are immature red blood cells that contain RNA and cytoplasmic remnants from earlier stages of development. A reticulocyte count provides information about bone marrow response and red blood cell production. There are four stages of reticulocyte maturation defined by their morphological appearance after staining. A reticulocyte count can be performed manually using supravital staining or automatically using flow cytometry to measure RNA levels. An increased reticulocyte count indicates bone marrow response to anemia while a decreased count suggests impaired red blood cell production.