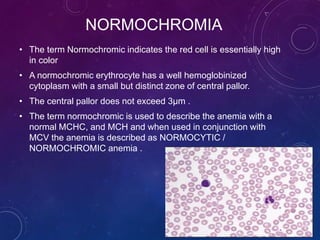
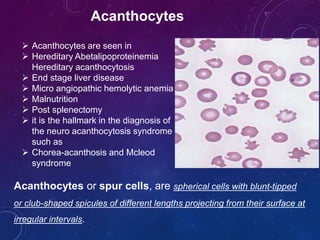
The document provides information about peripheral blood smear examination. It discusses how peripheral blood smears are an important diagnostic tool that provide information about hematologic disorders through examination of red blood cells, white blood cells, and platelets. It outlines the procedure for preparing, staining, and examining a peripheral blood smear under the microscope. Key things examined include red blood cell size, shape, color, and inclusions, as well as white blood cell and platelet counts, differentials, and morphologies. Common red blood cell abnormalities seen include microcytosis, macrocytosis, anisocytosis, poikilocytosis, hypochromasia, polychromasia, and inclusions.