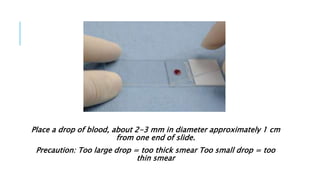
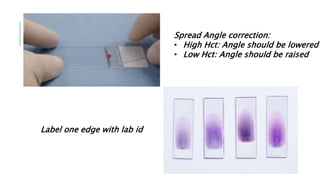
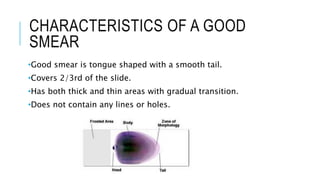
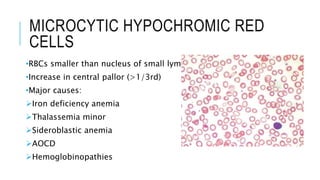
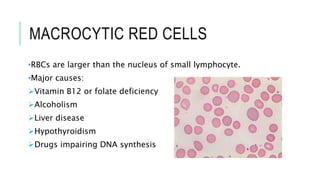
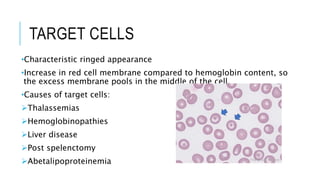
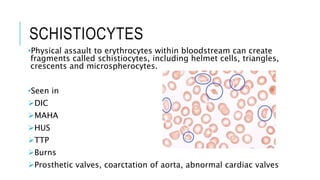
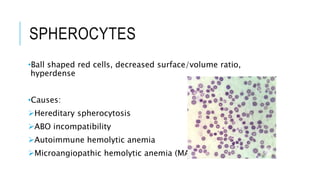
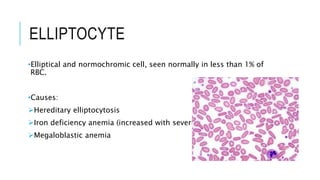
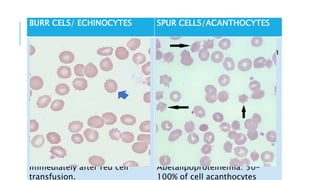
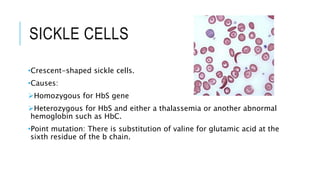
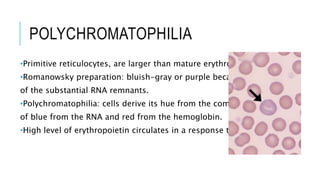
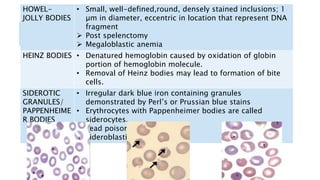
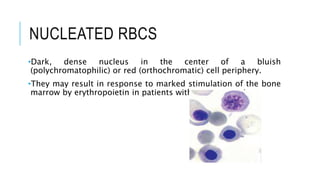
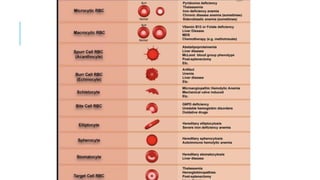
The document outlines the role of peripheral blood smears in evaluating anemia, thrombocytopenia, and abnormal cells, along with detailed techniques for preparing and staining the smears. It describes various red blood cell morphological abnormalities and inclusions that may be observed, their causes, and significance in diagnosing different conditions. Additionally, the document provides insights into the characteristics of a good smear and specific staining methods essential for accurate analysis.