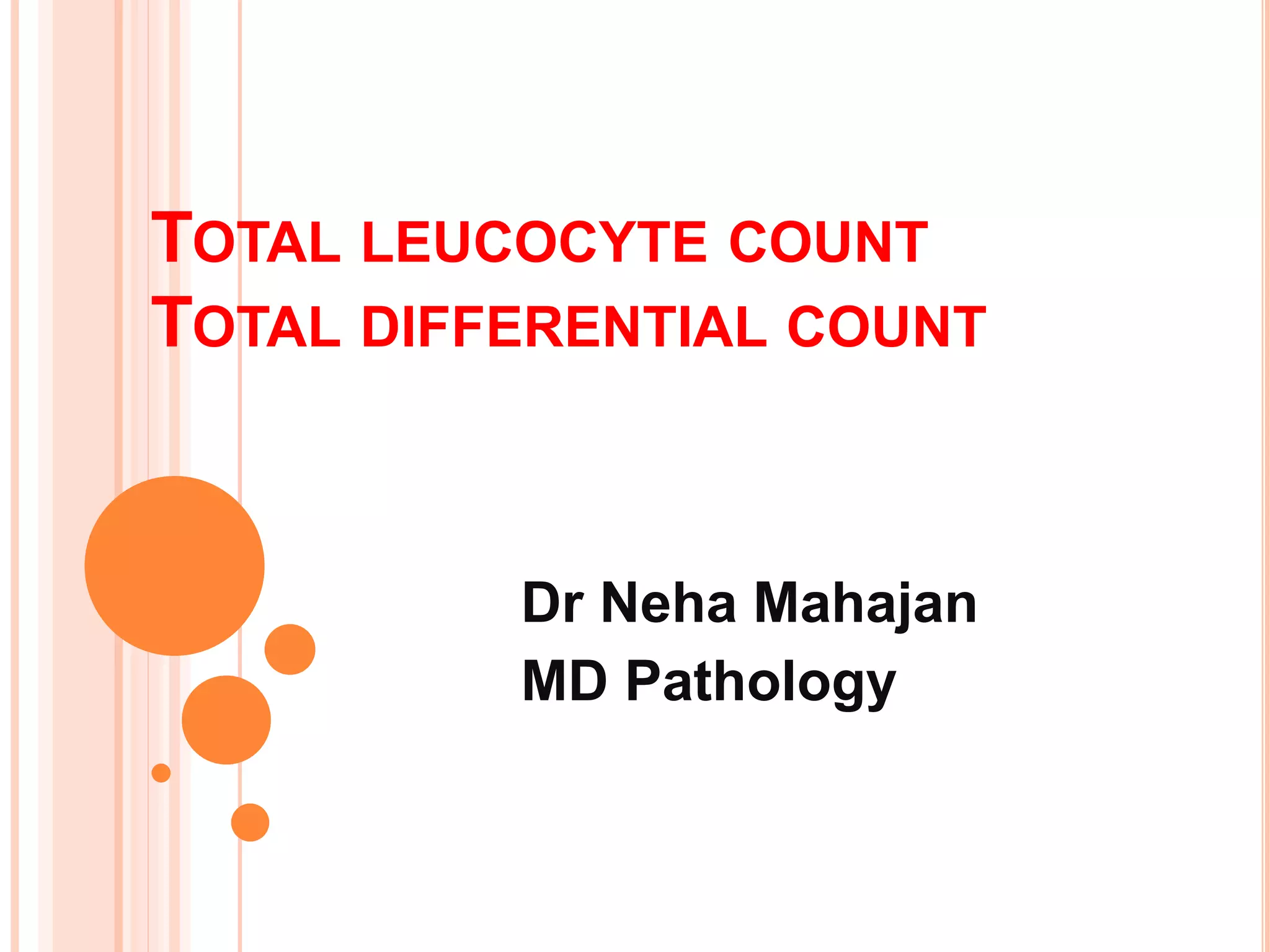
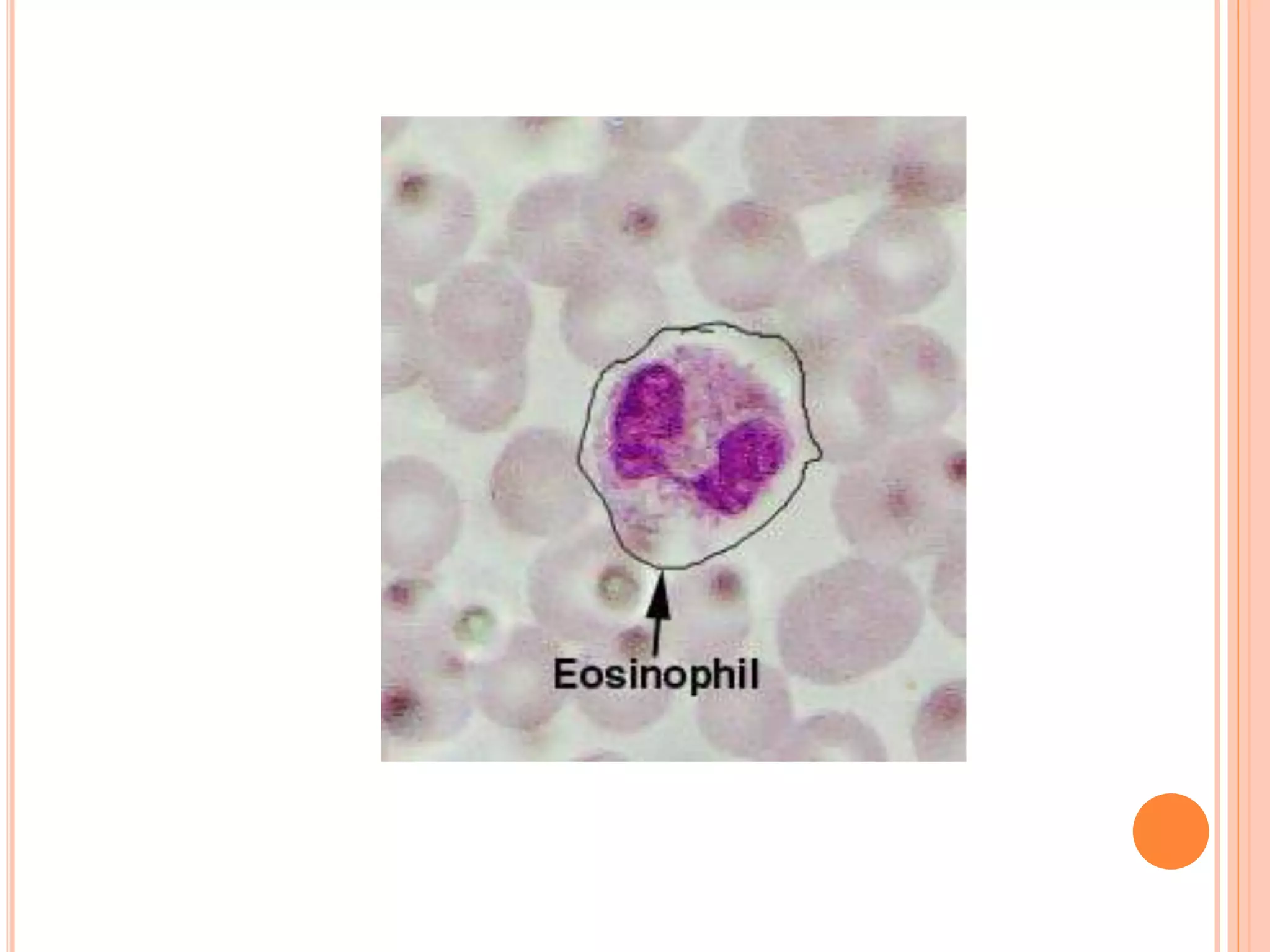
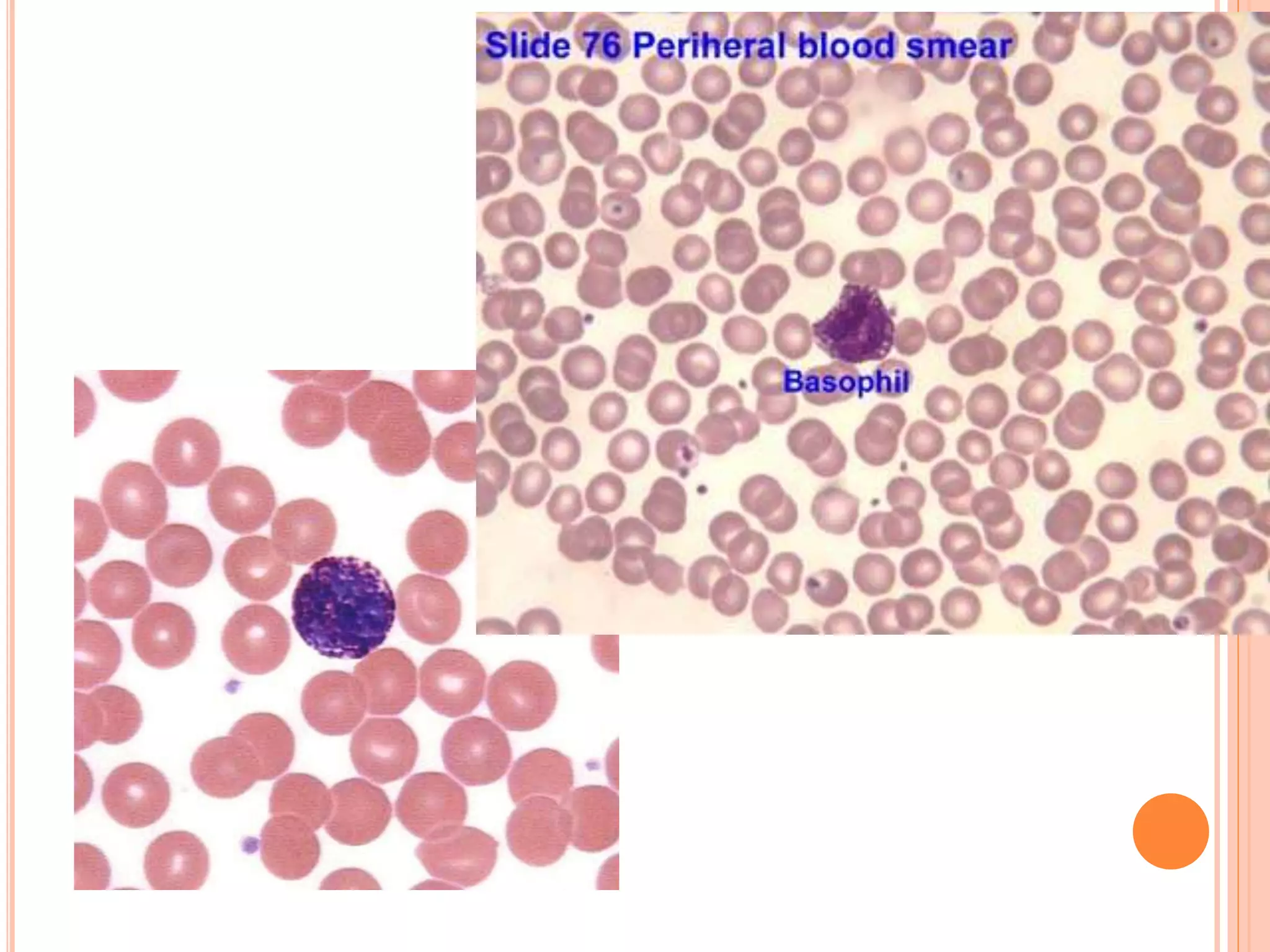
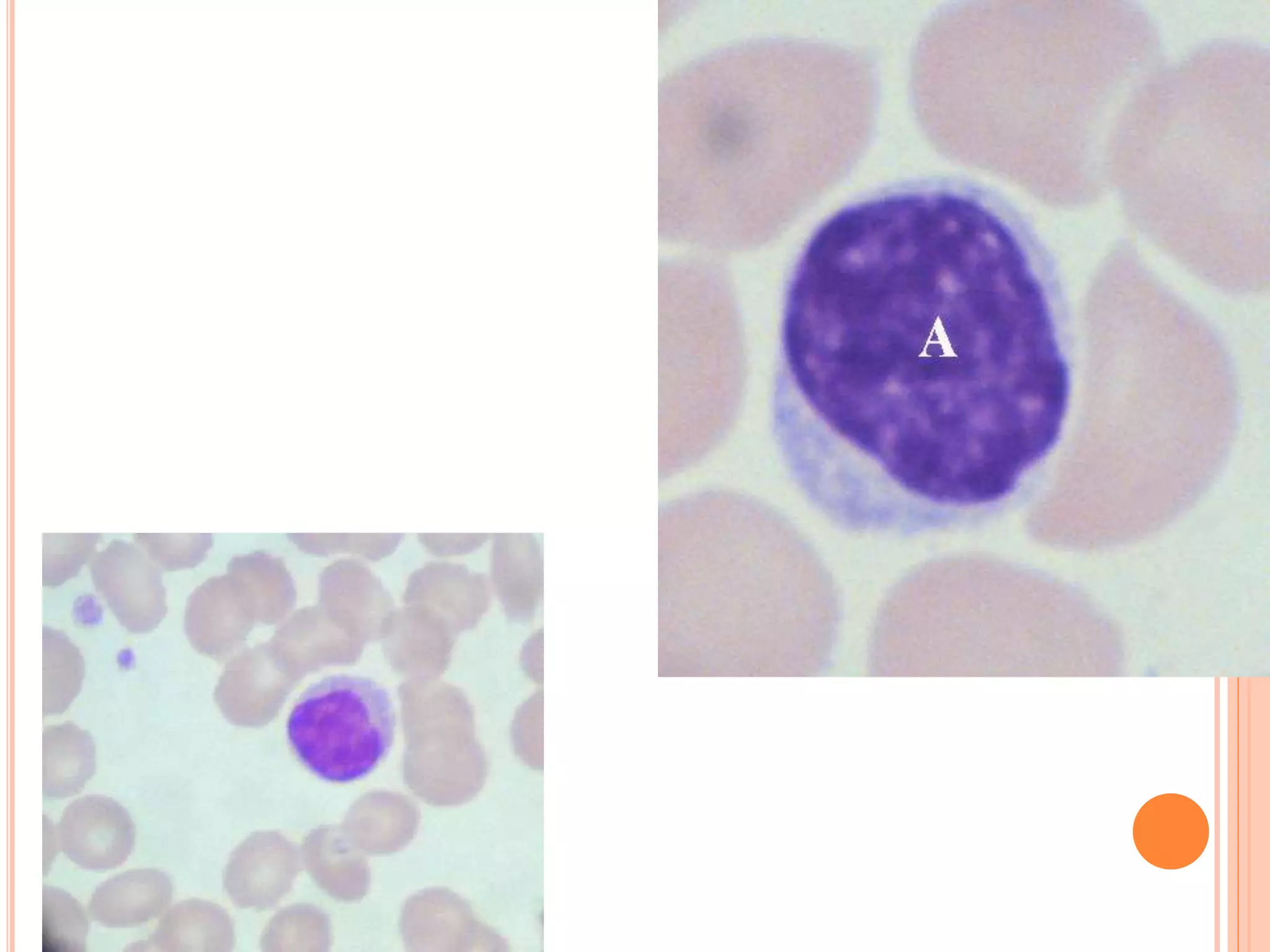
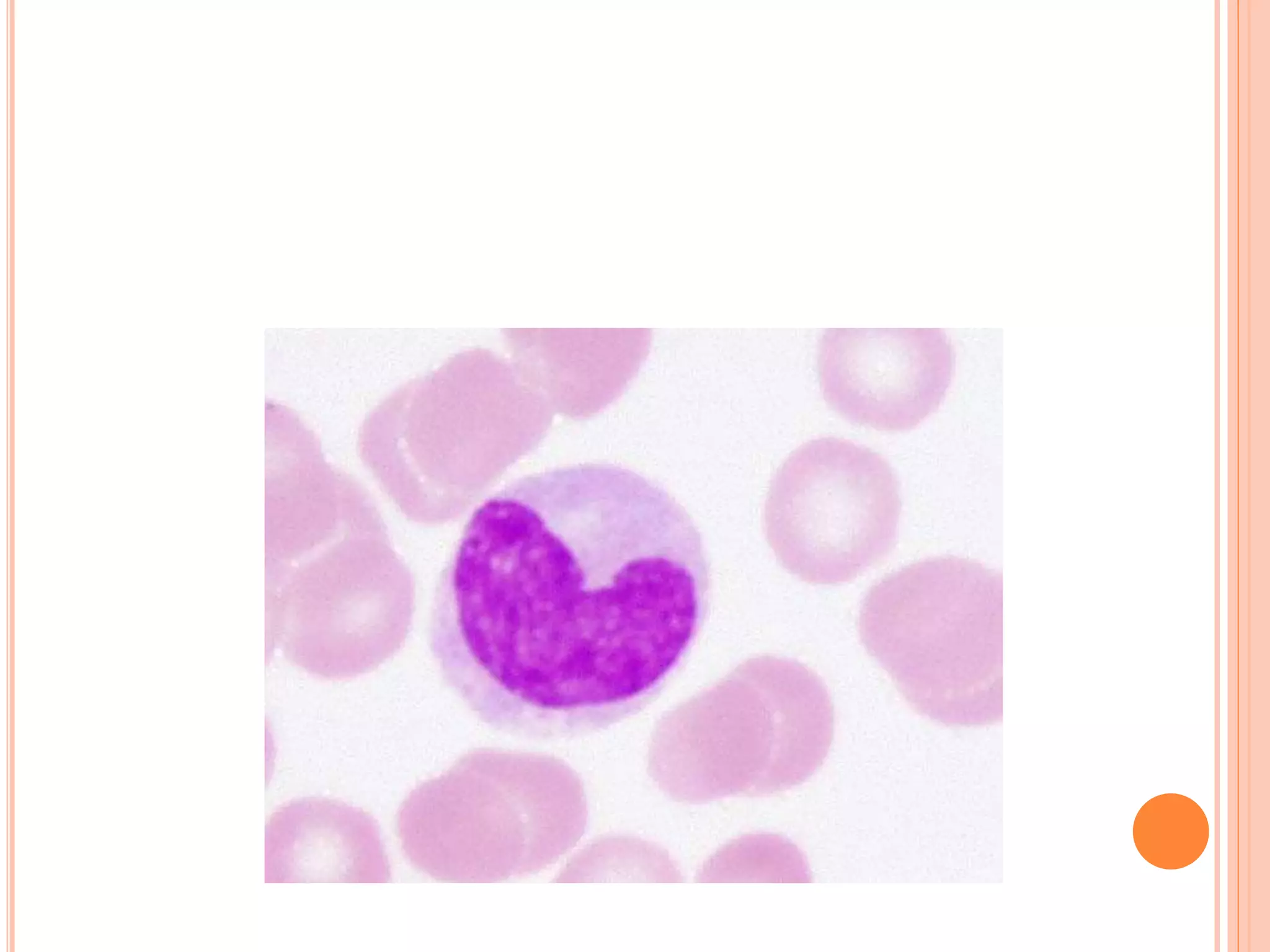
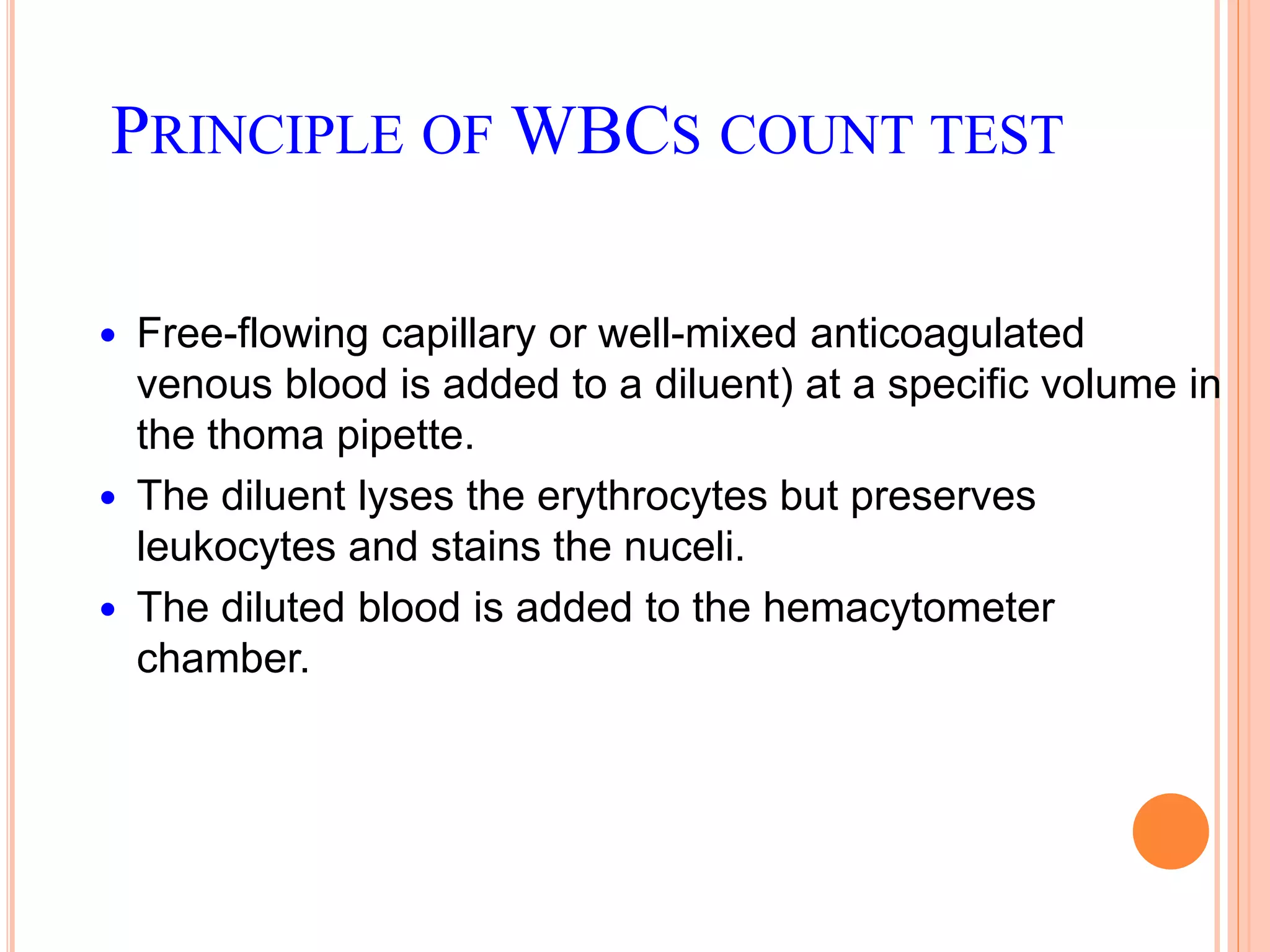
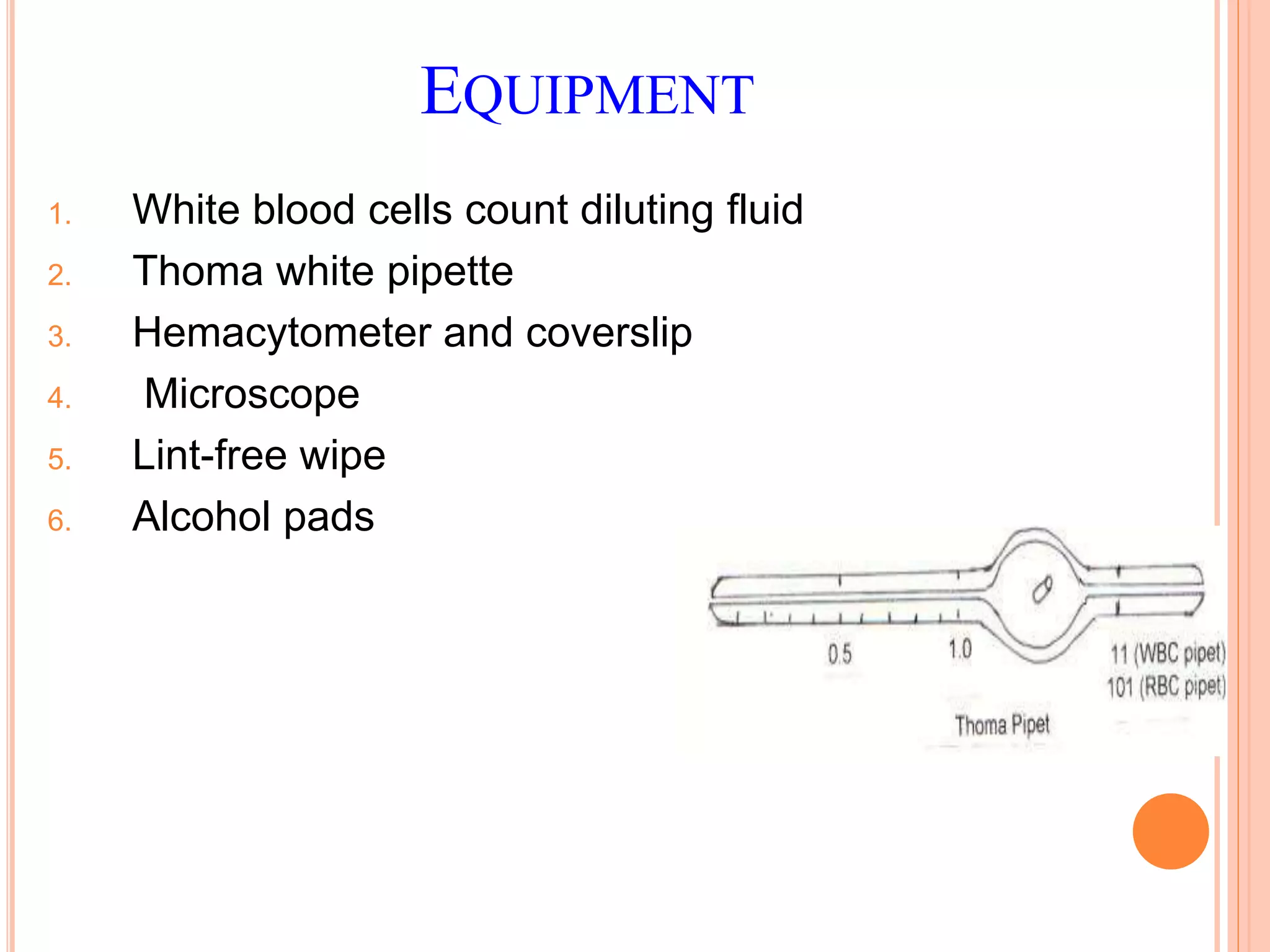
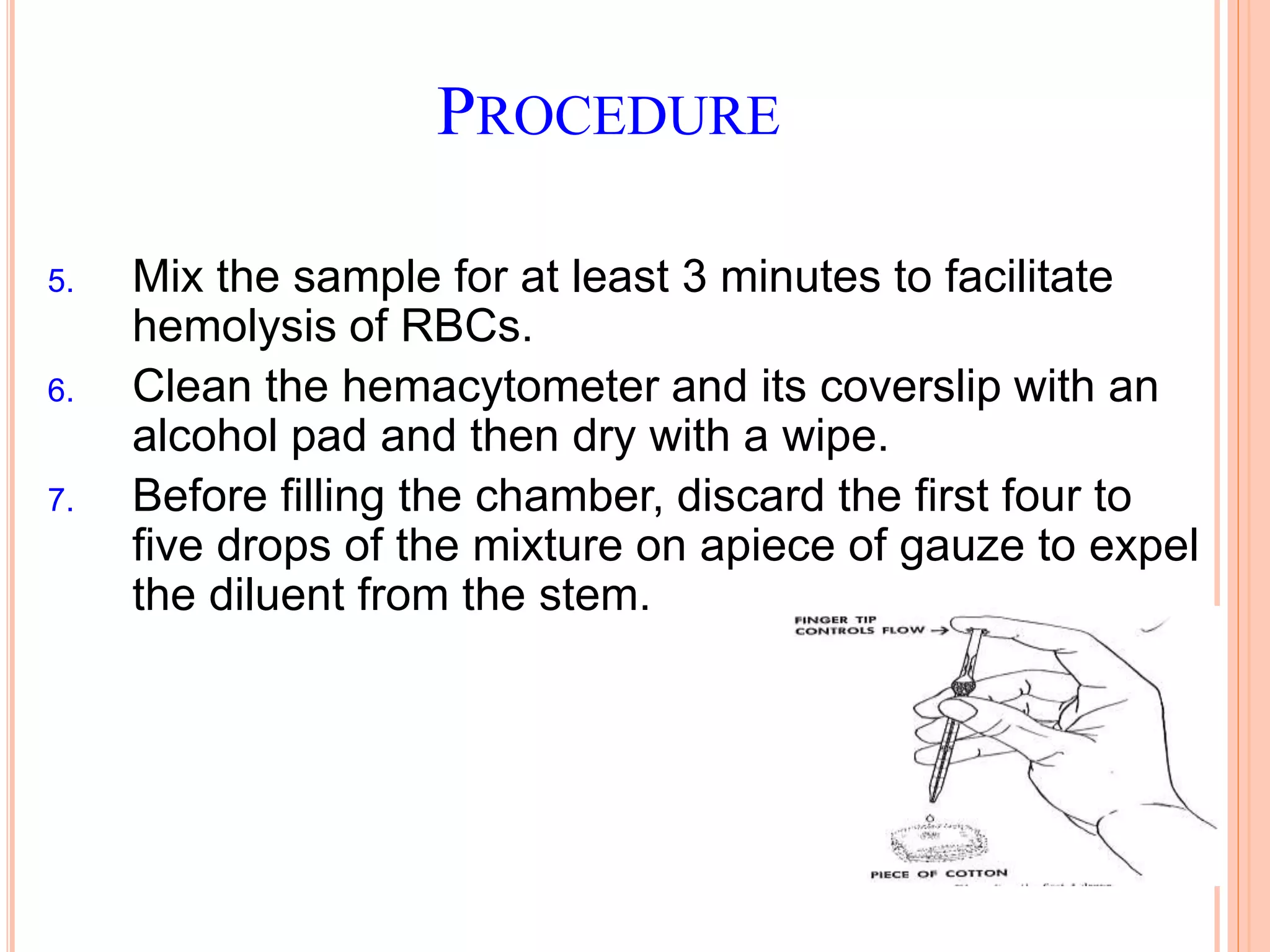
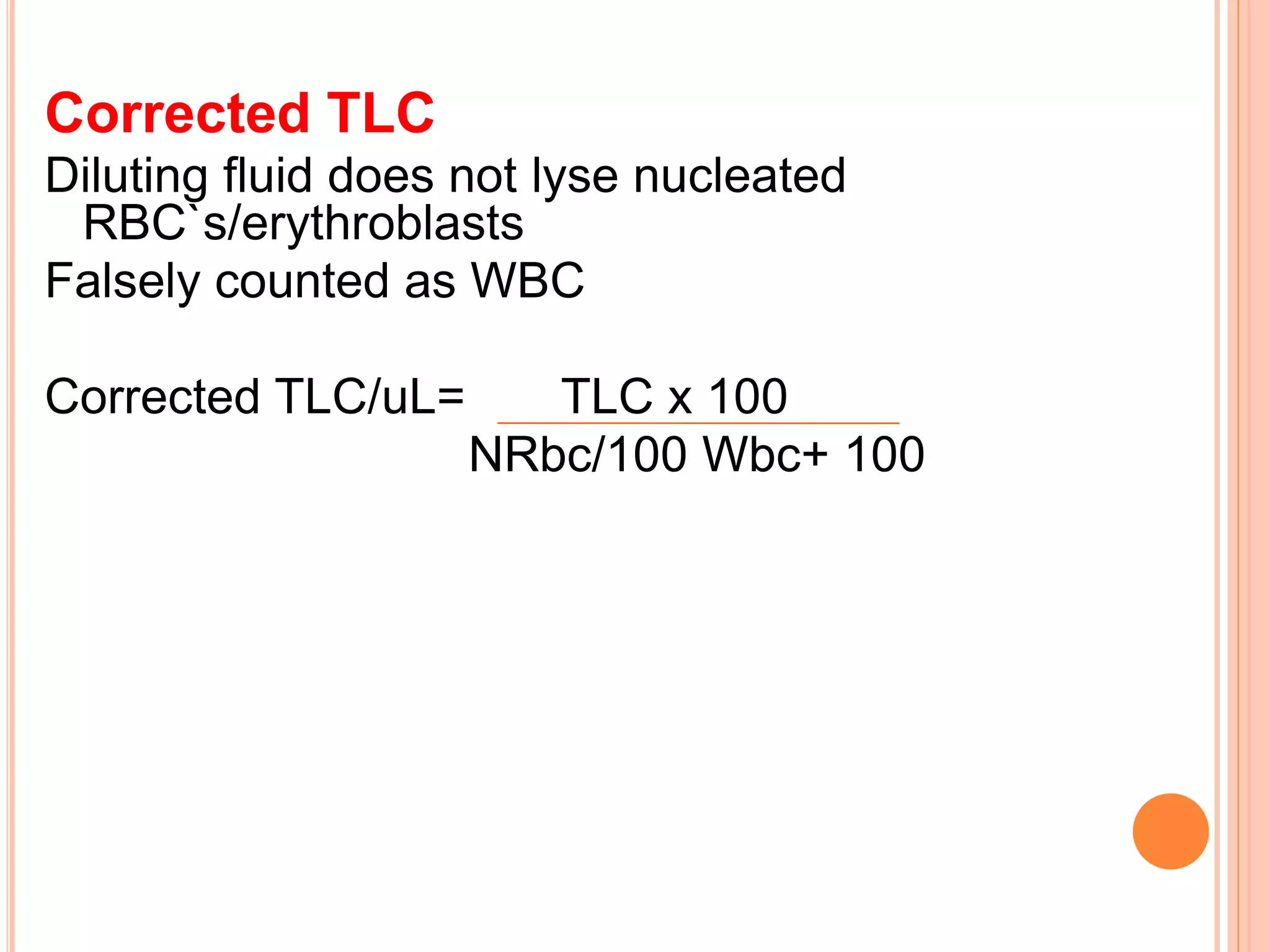
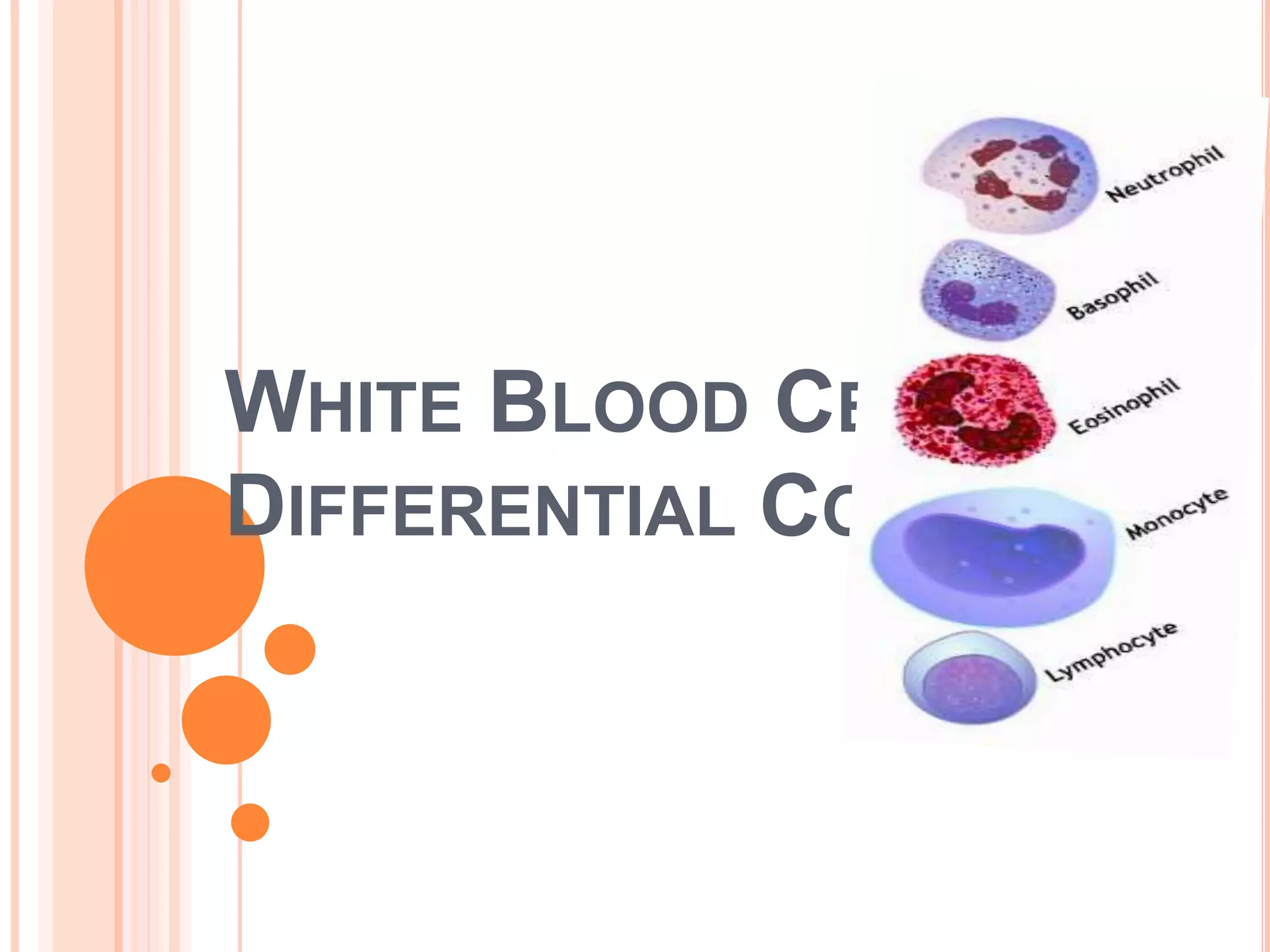
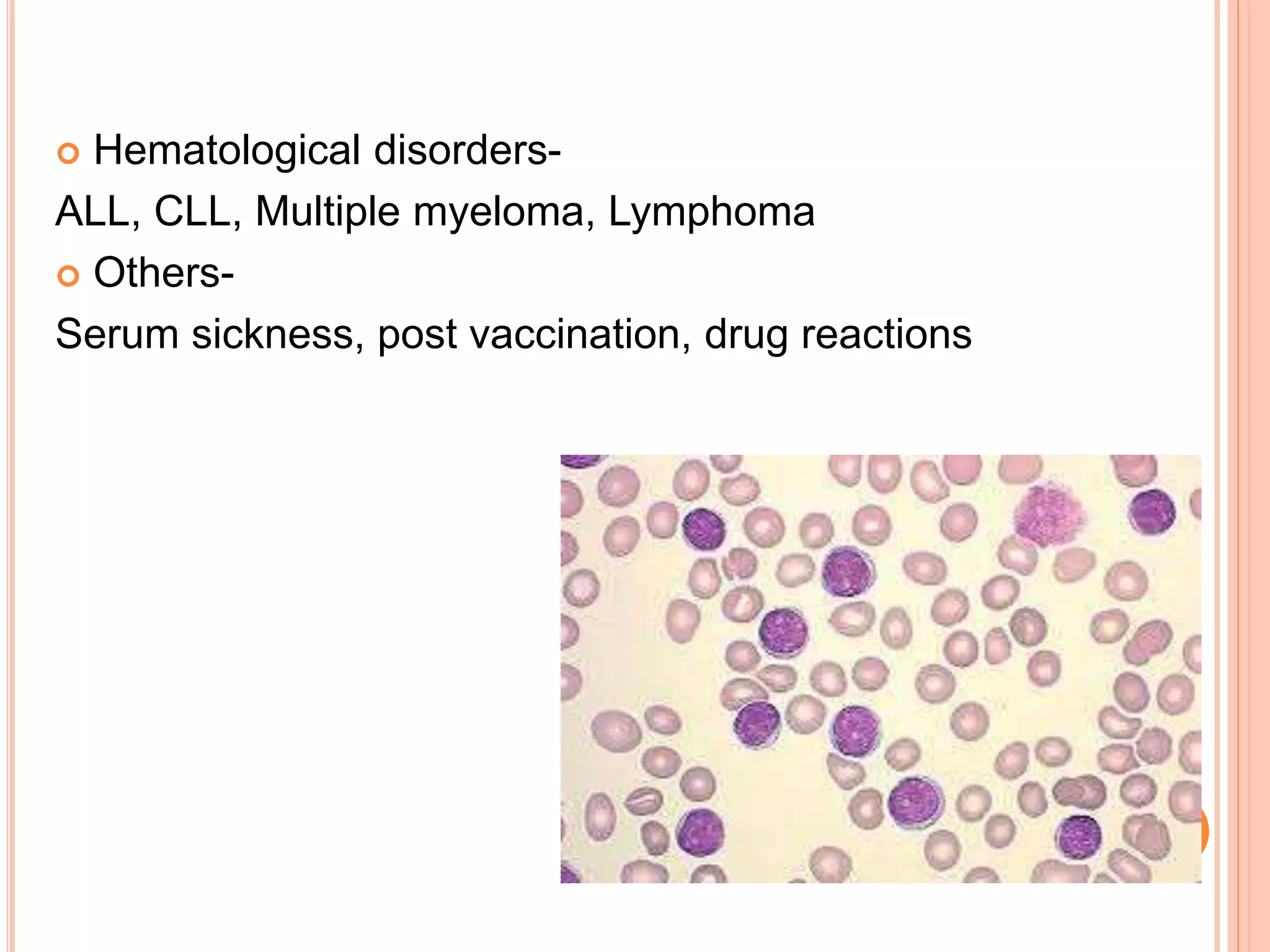
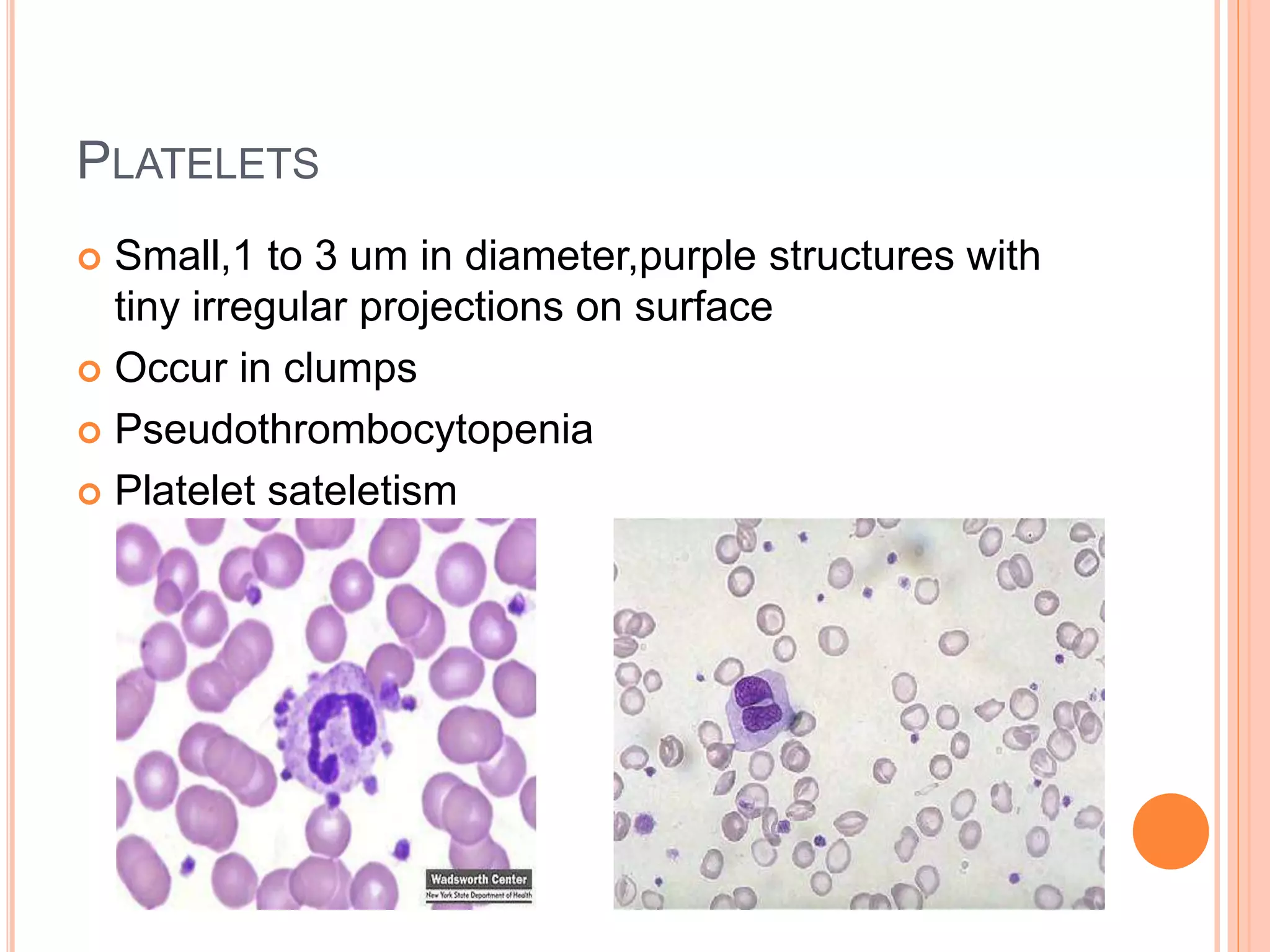
This document discusses total leukocyte count and differential counting. It describes the types of white blood cells including granulocytes (neutrophils, eosinophils, basophils) and agranulocytes (lymphocytes, monocytes). The procedure for a total leukocyte count involves diluting a blood sample and counting cells under a microscope. A differential count identifies percentages of each type of white blood cell by examining stained blood smears under oil immersion lens. Normal ranges and abnormal findings are also outlined.