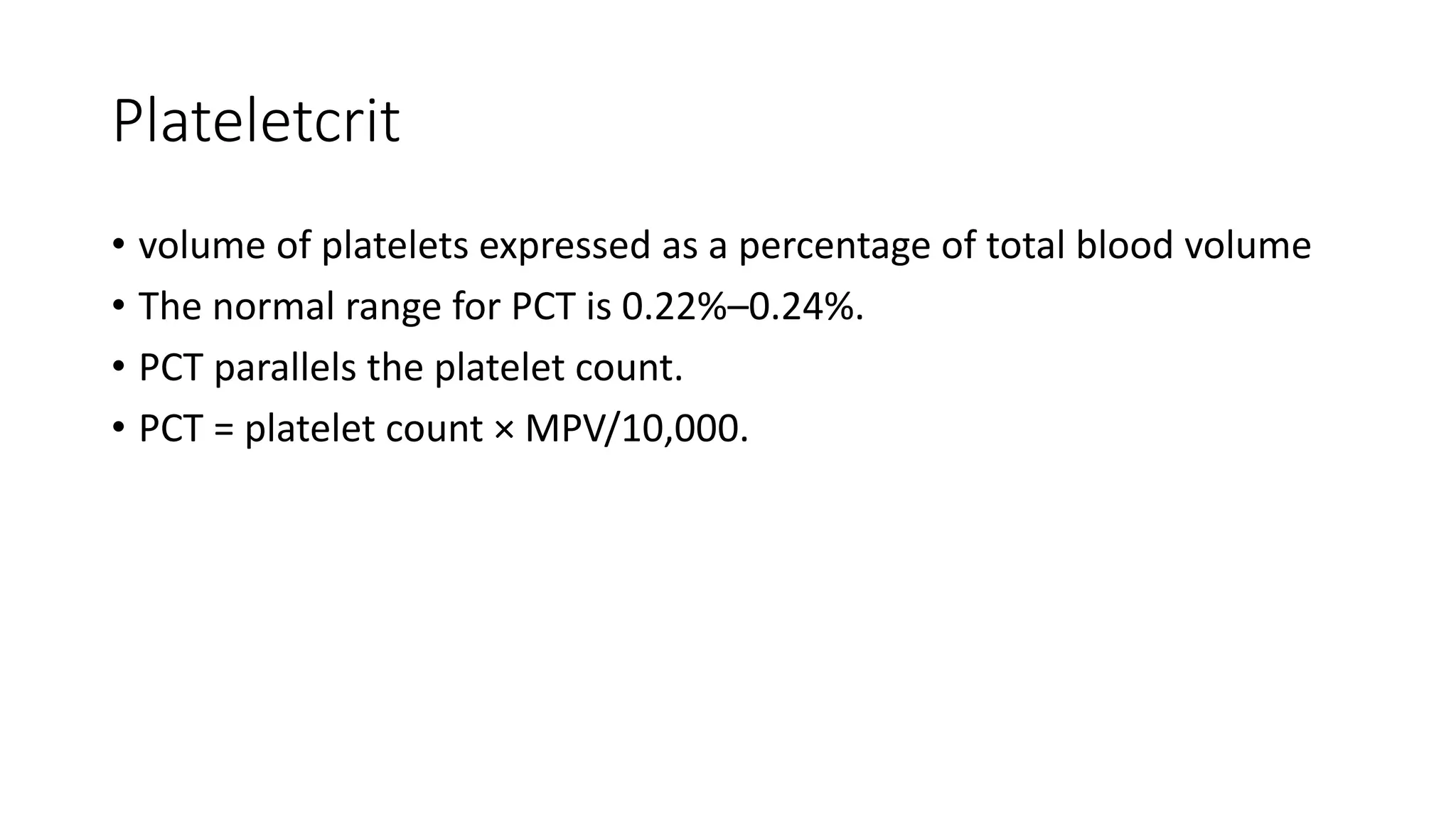
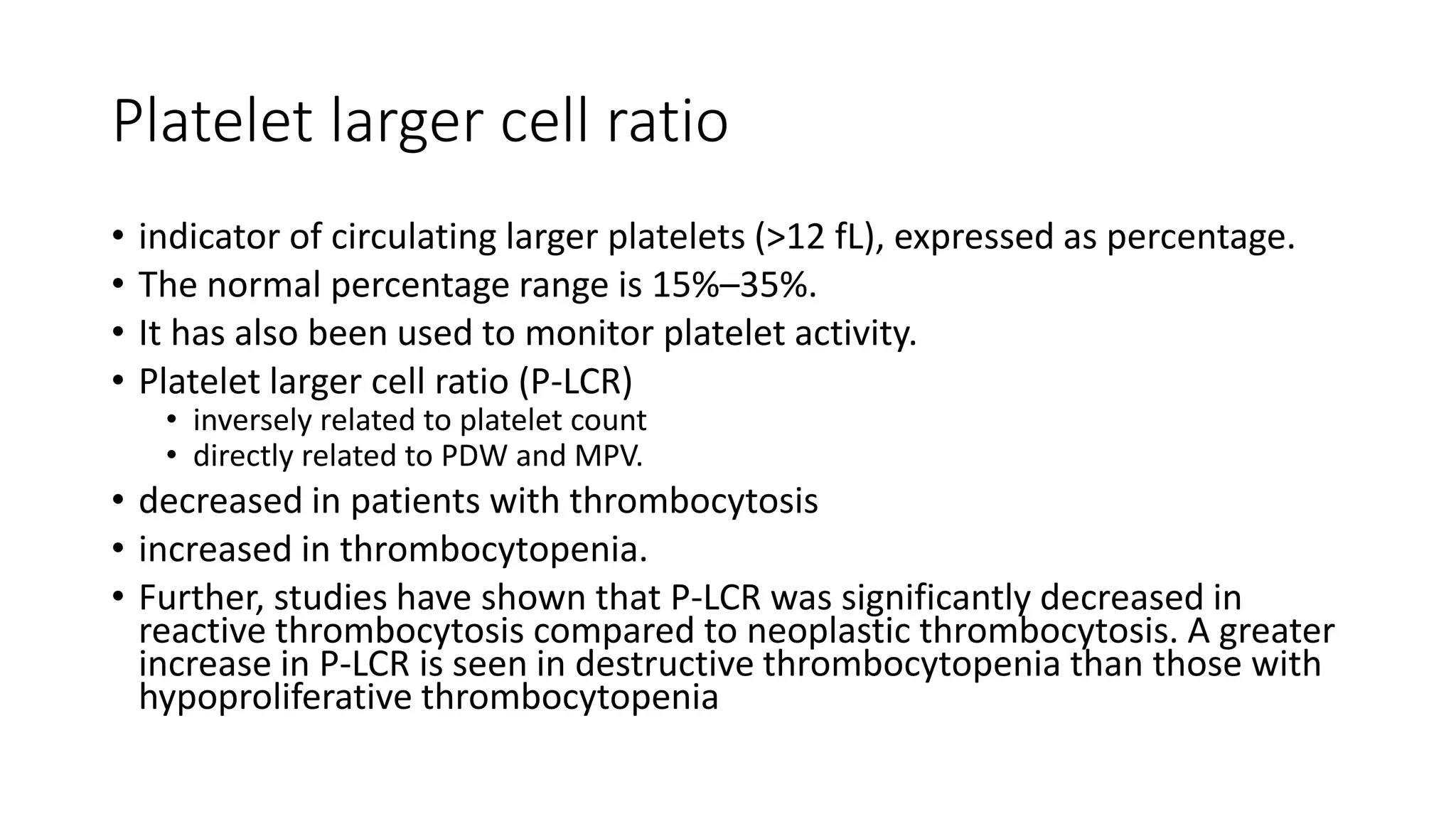
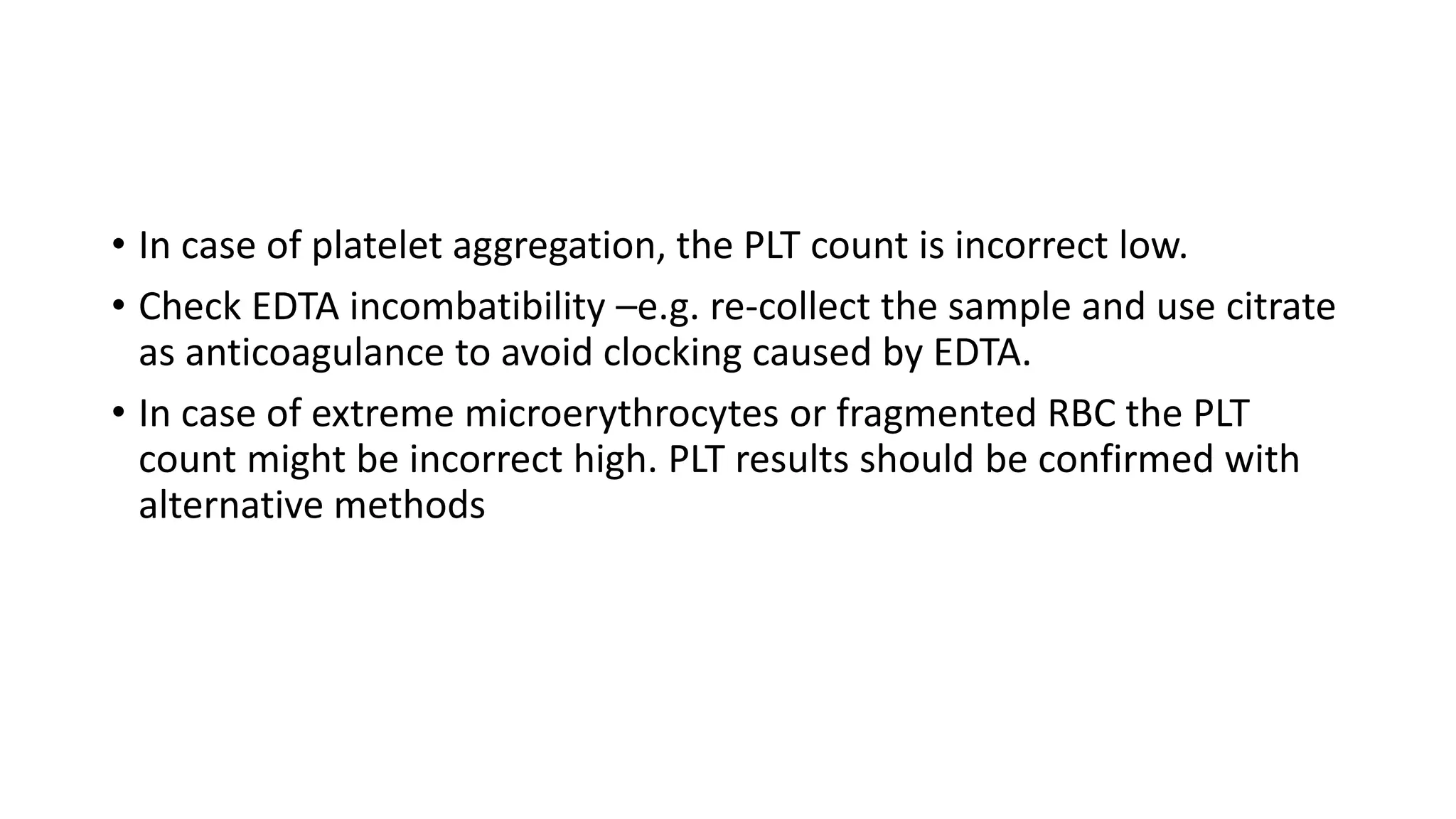
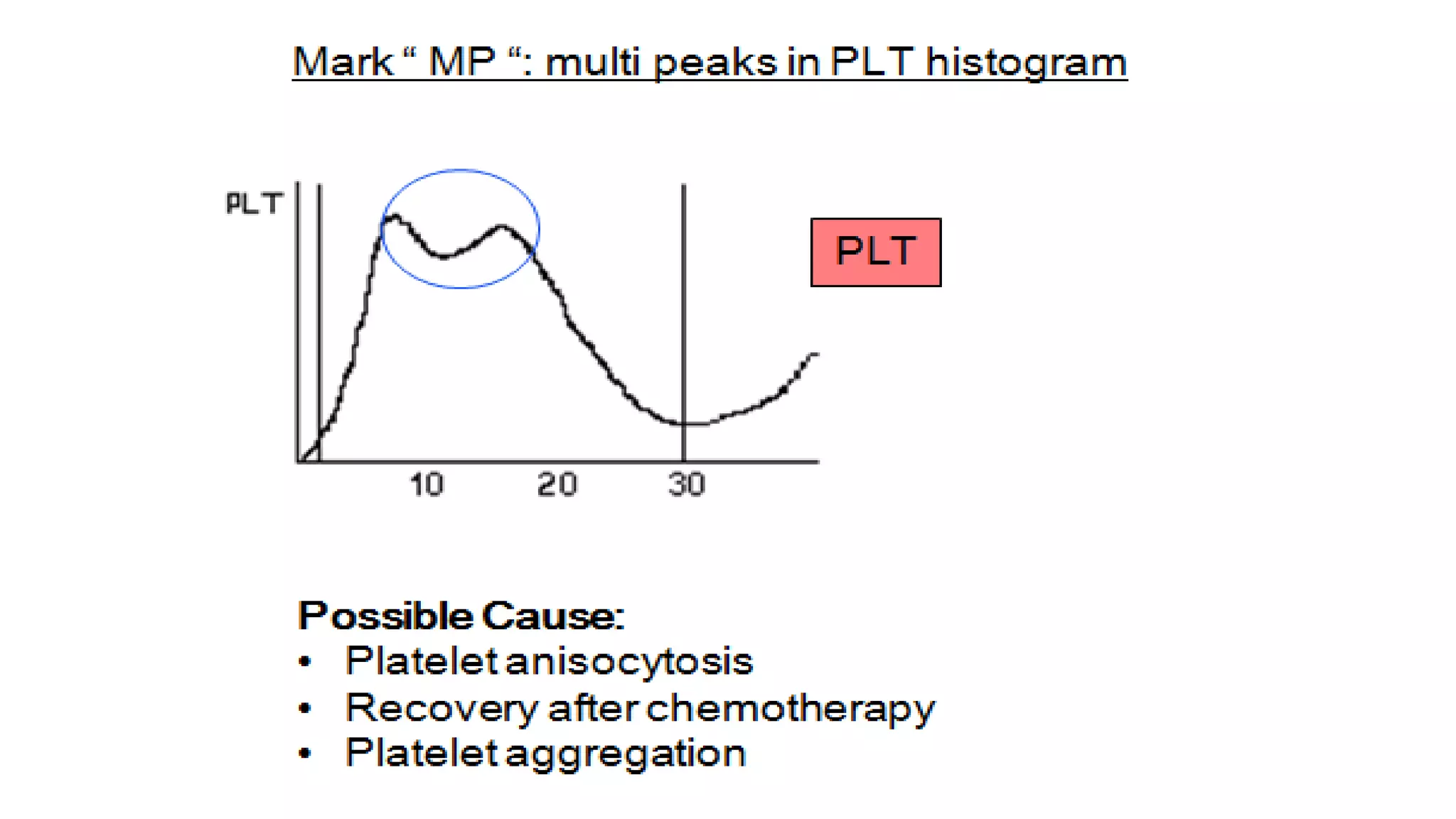
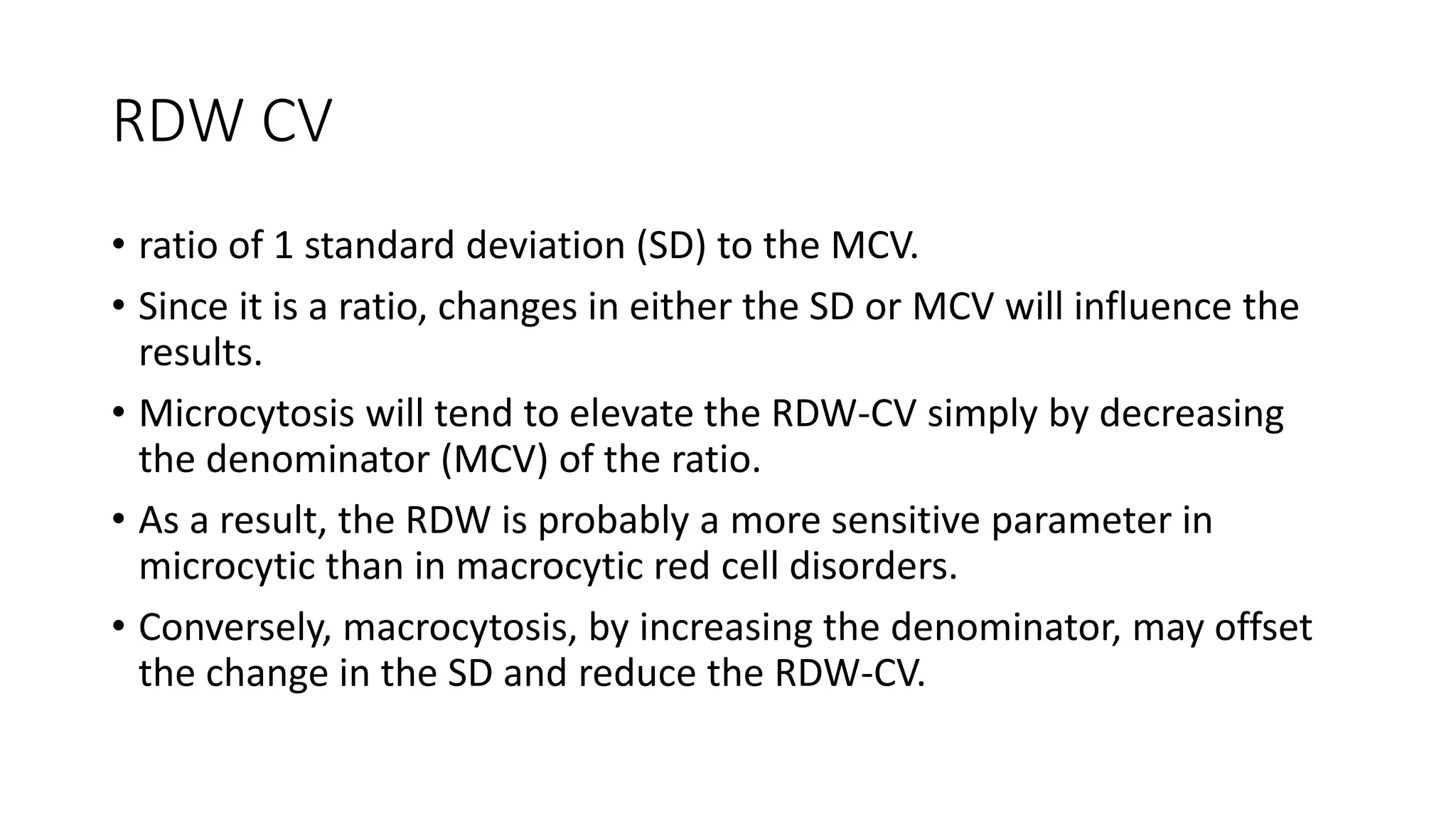
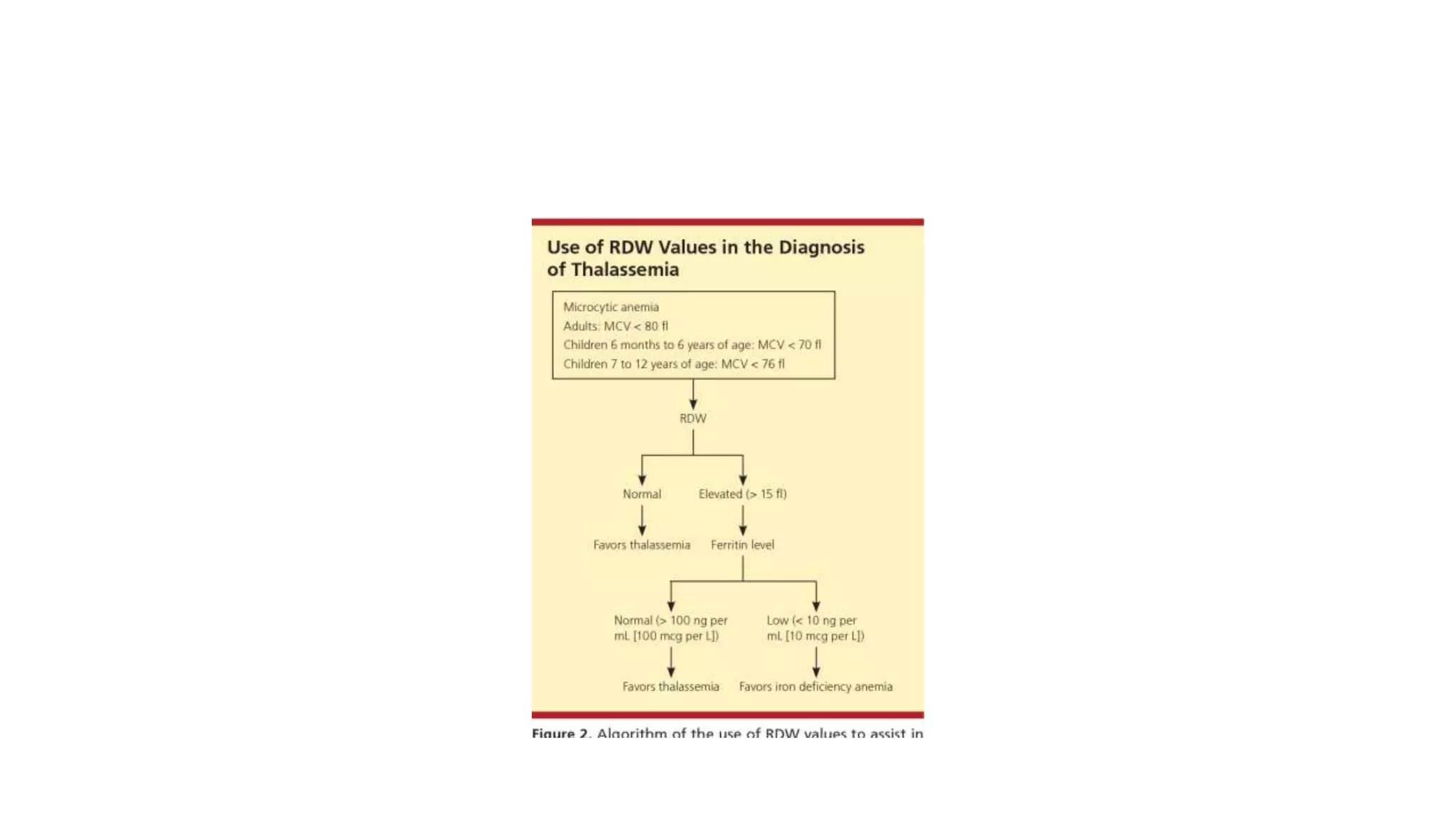
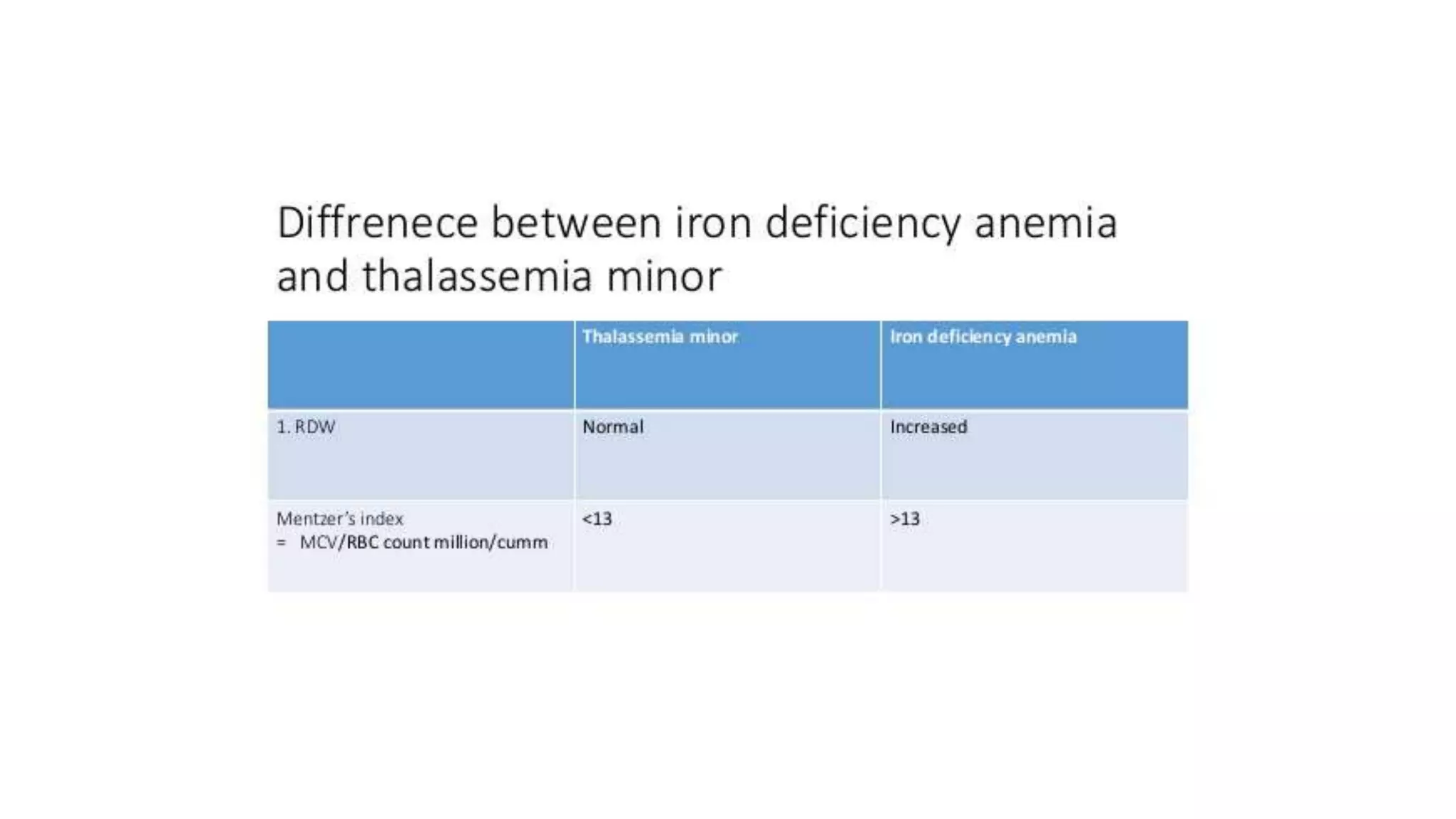
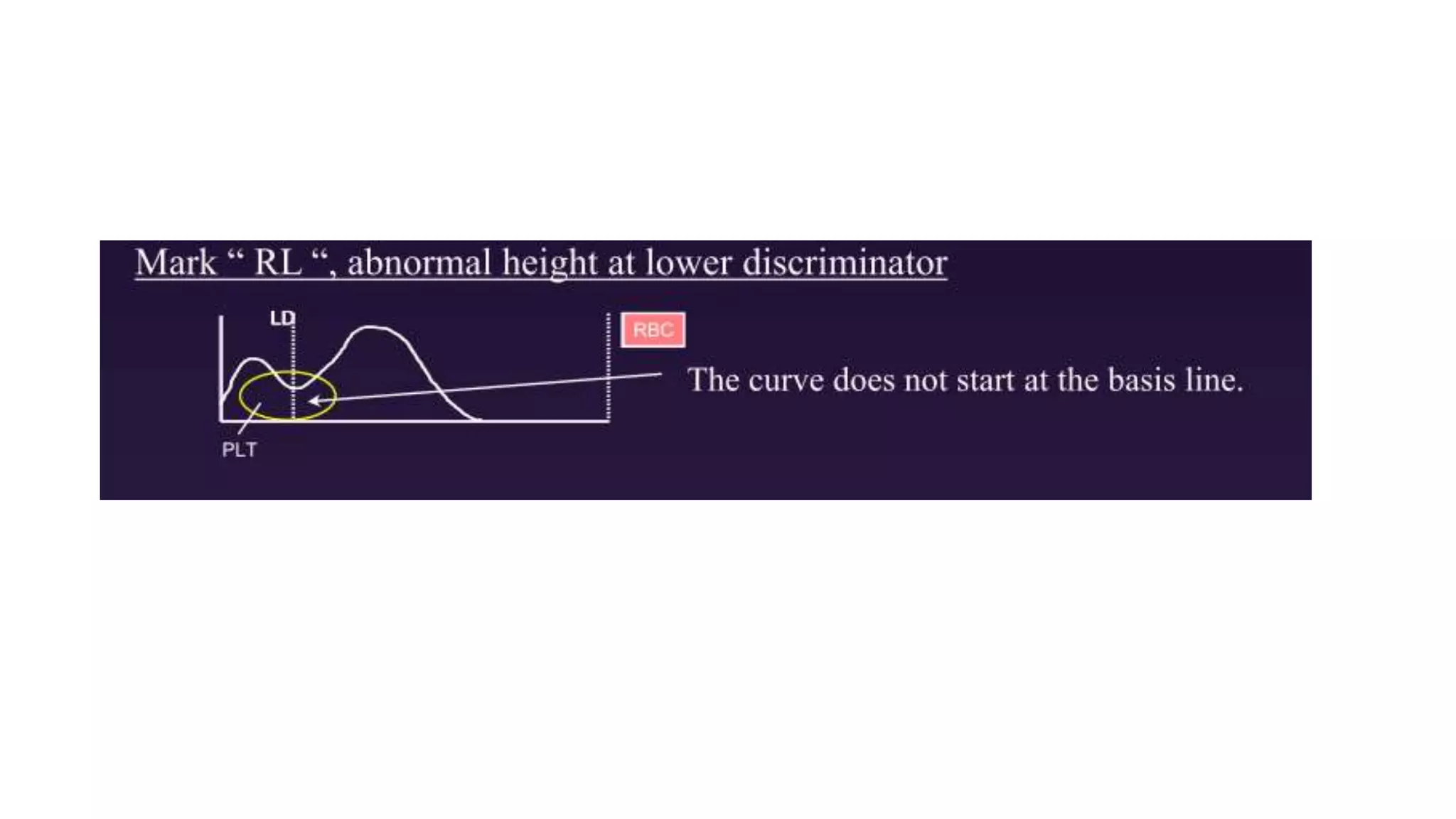
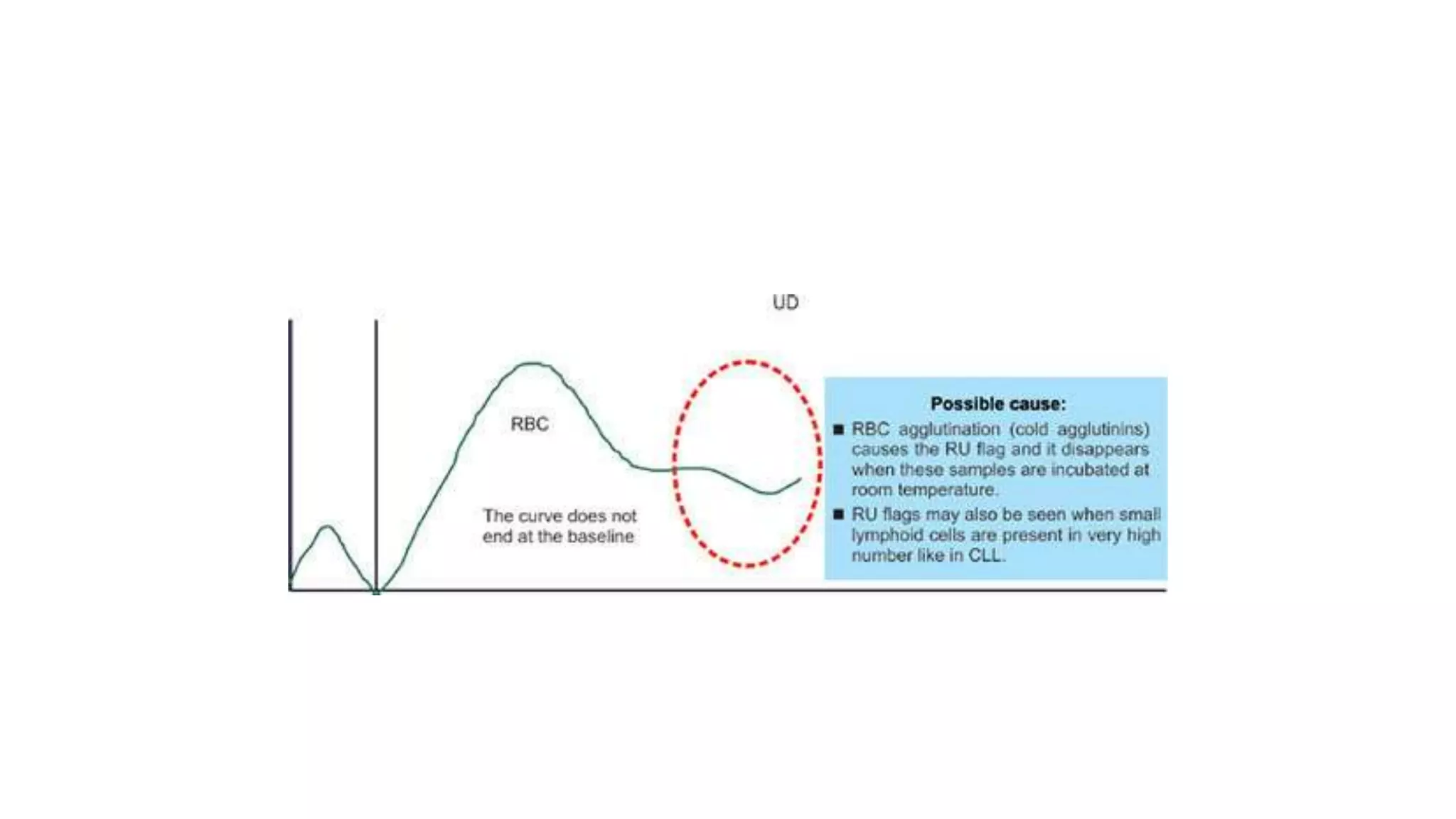
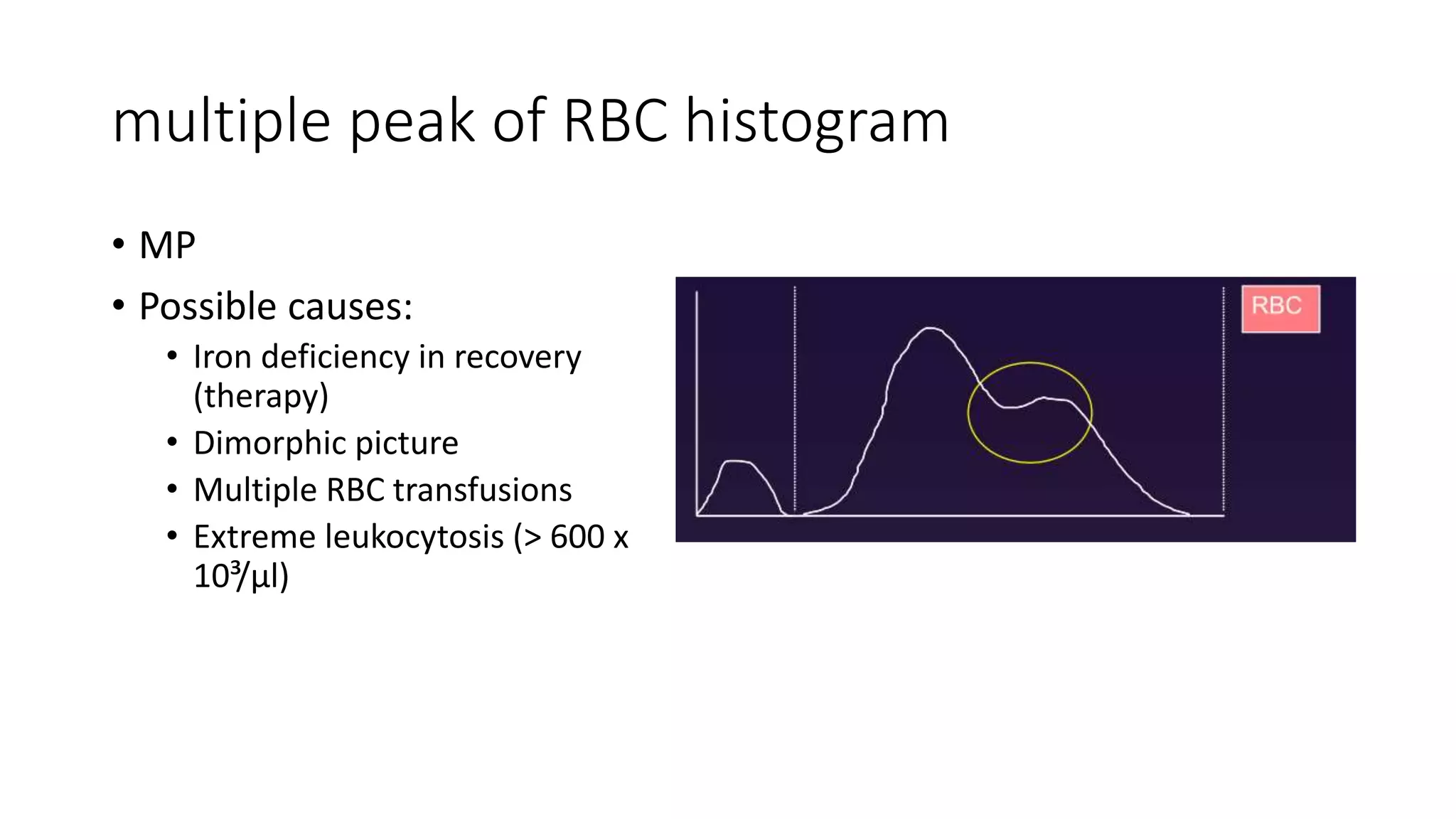
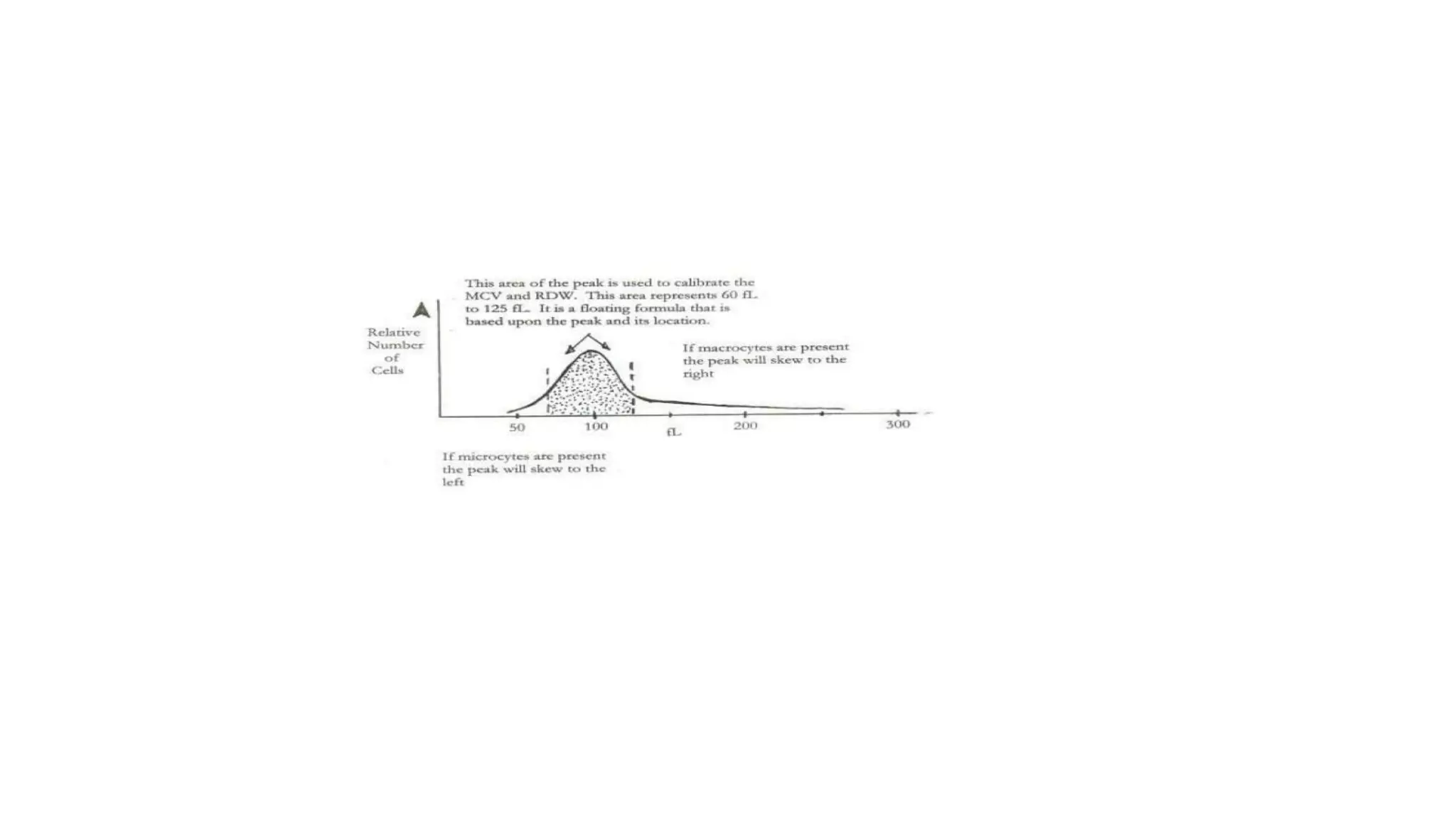
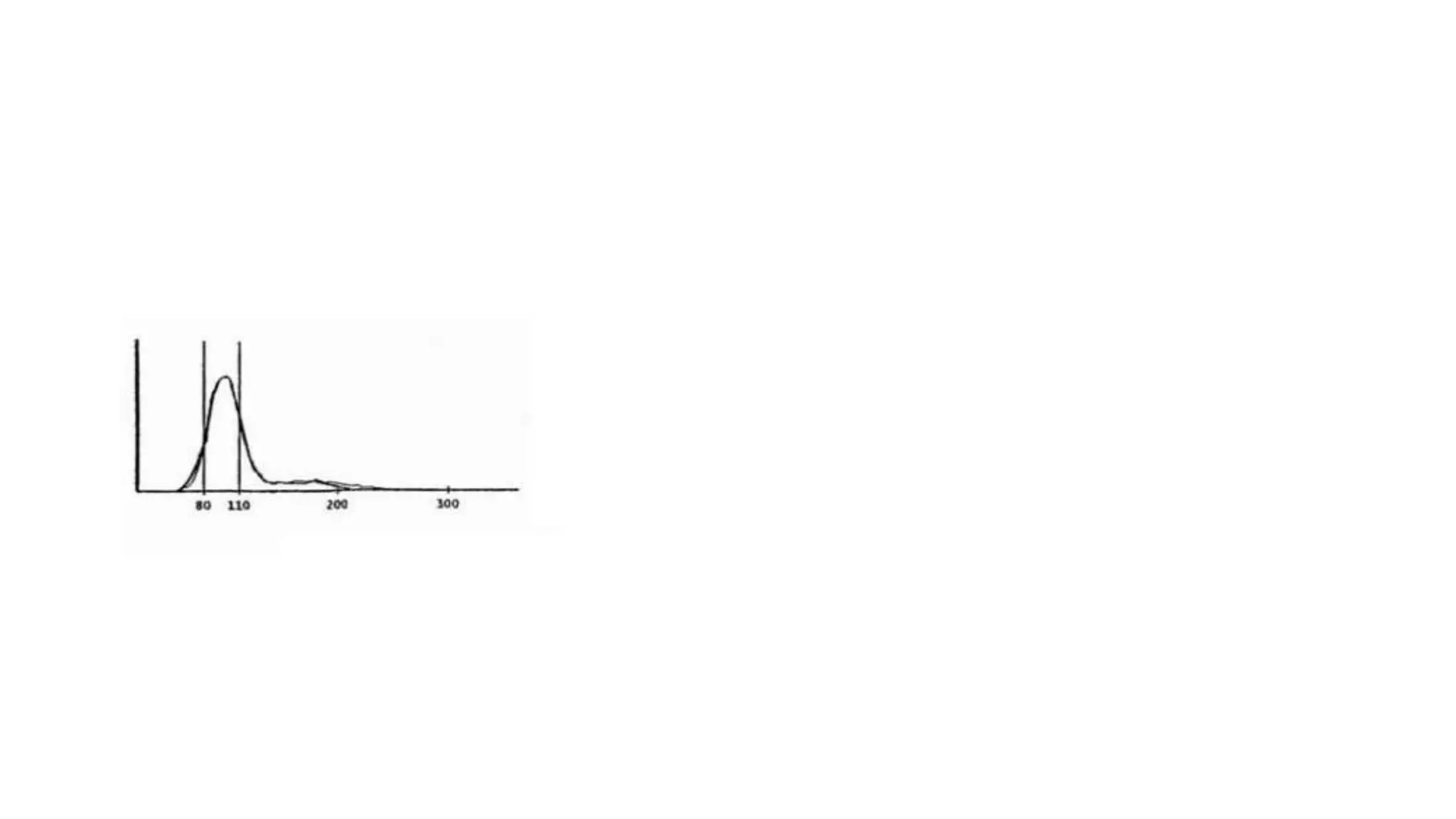
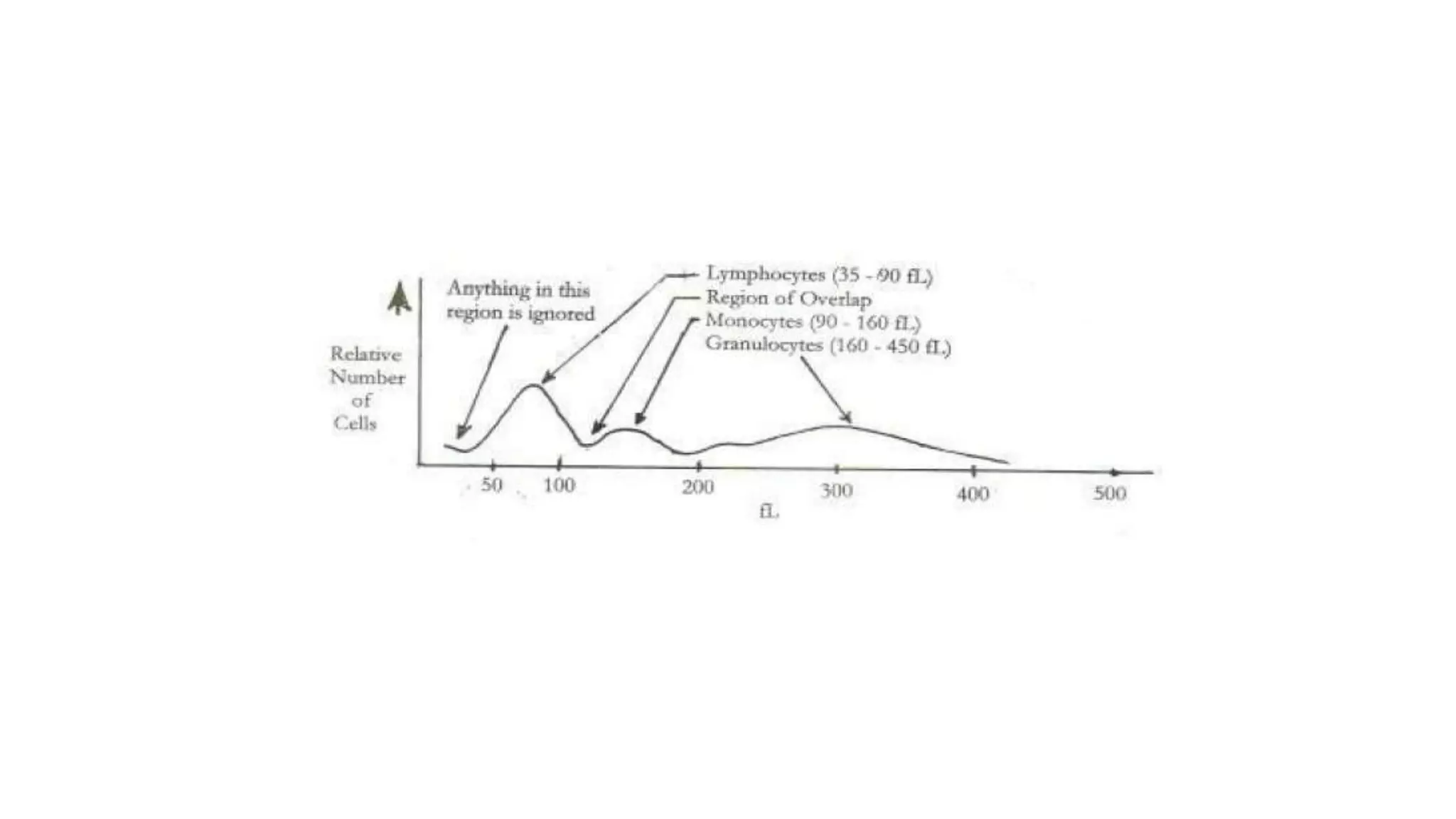
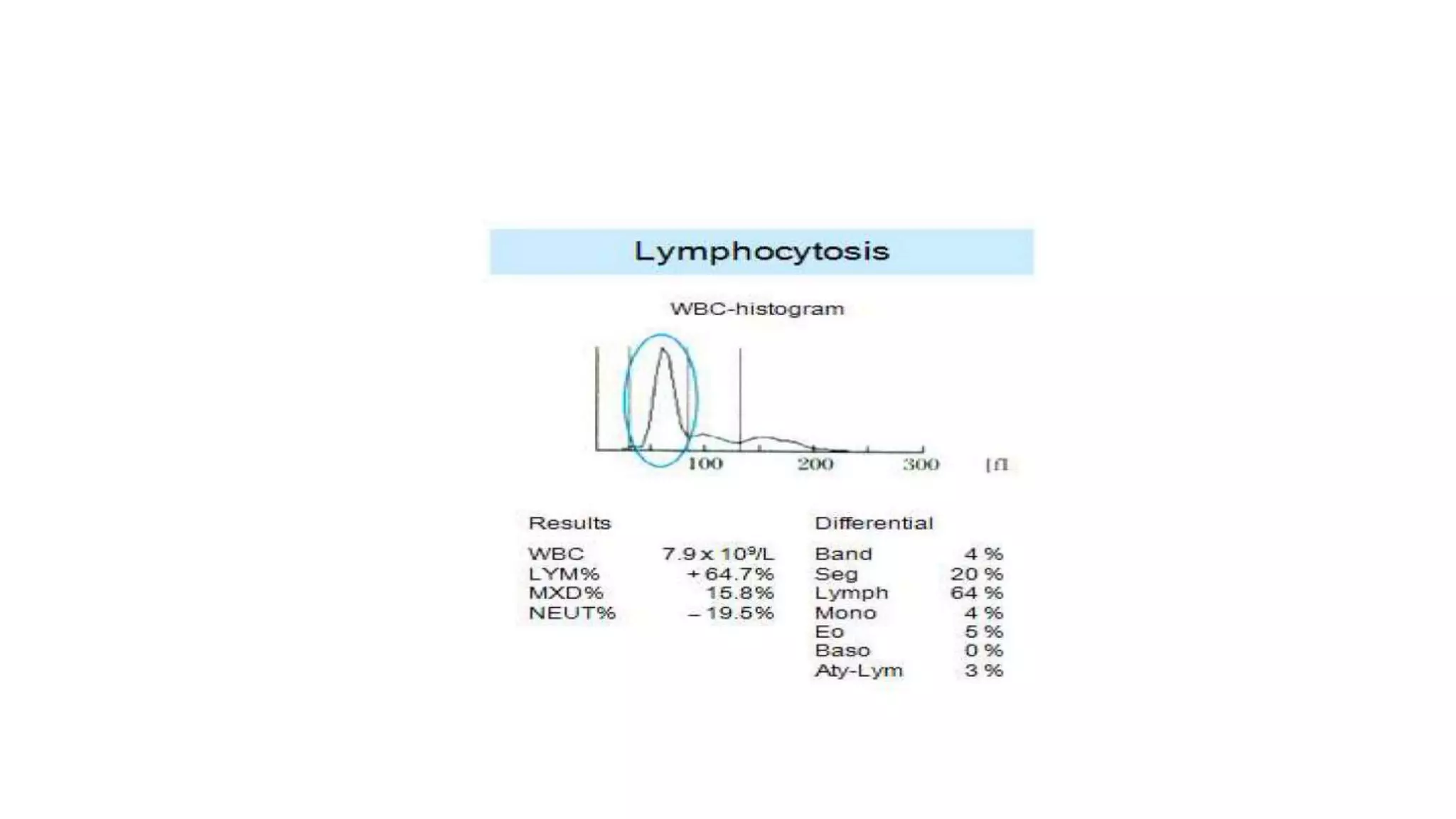
- The document discusses histograms generated by cell counters that graphically represent cell population data. It focuses on histograms for red blood cells (RBCs), white blood cells (WBCs), and platelets.
- Key parameters for each type of histogram are defined, such as how cells are counted and measured, normal ranges, and flags that indicate potential issues. Common causes for shifts or abnormalities in the histograms are also outlined.
- The information provides guidance on interpreting cell counter histograms and histograms to help evaluate a patient's blood cell counts and detect possible blood disorders or interferences.





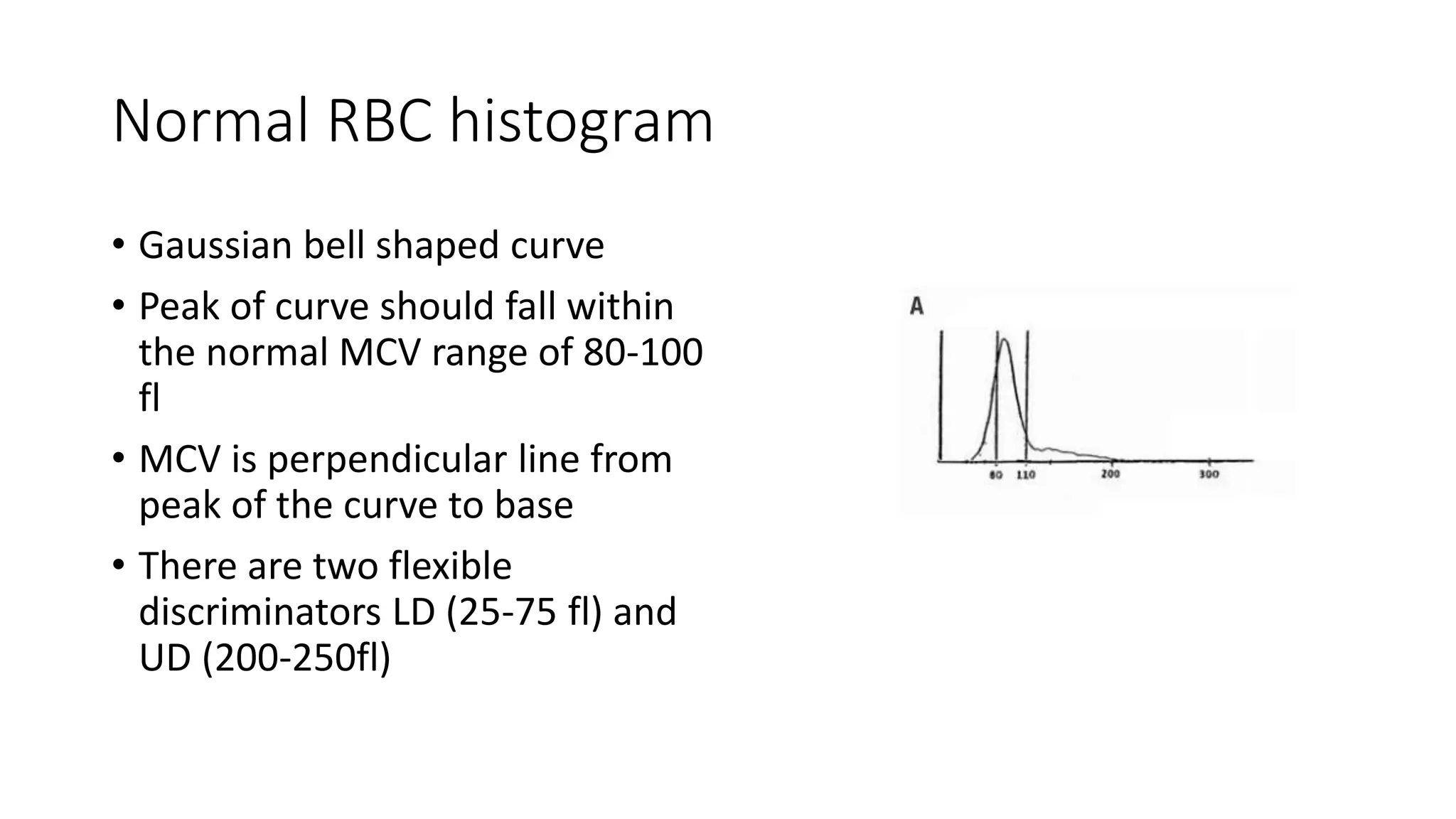







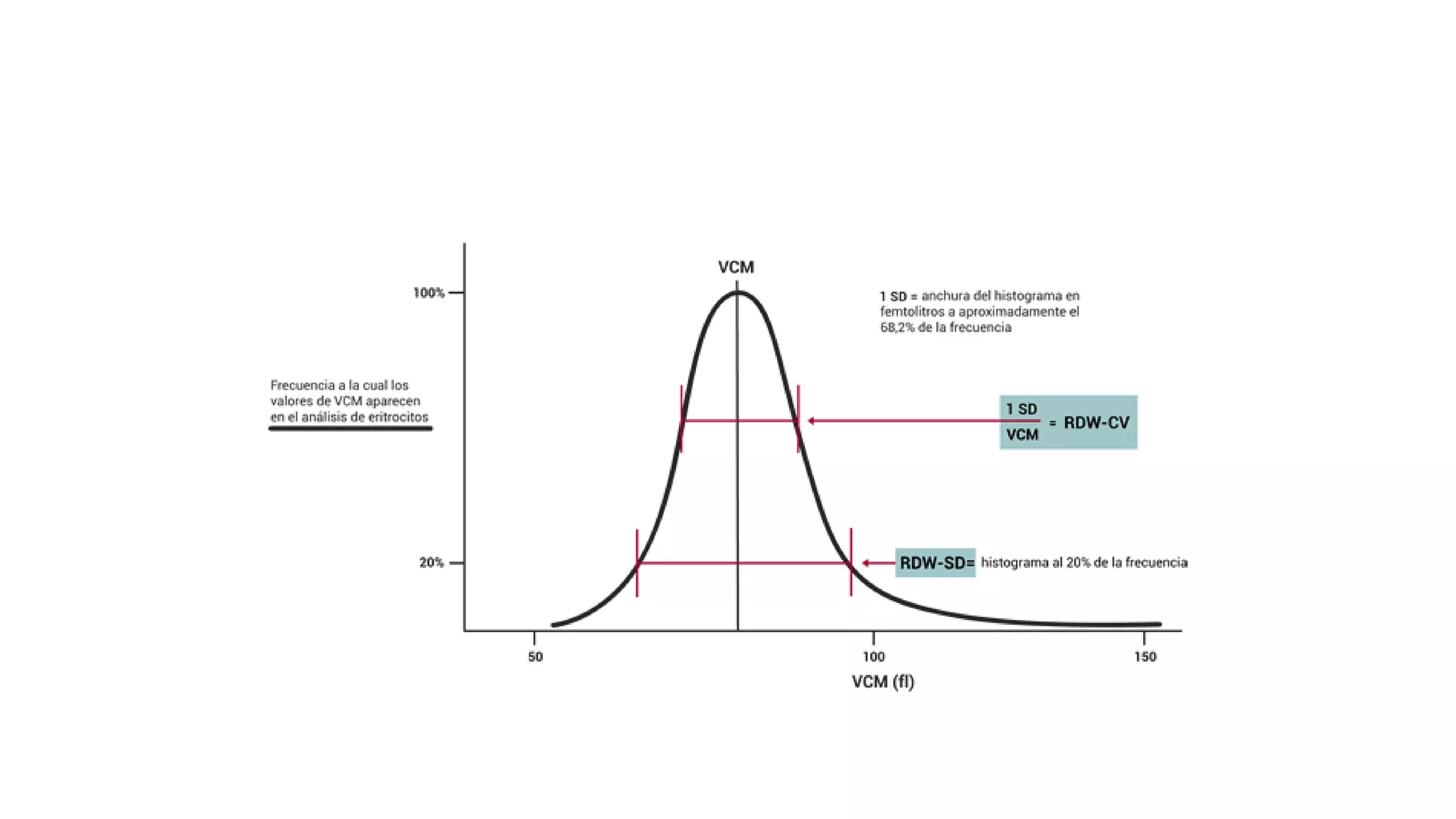
























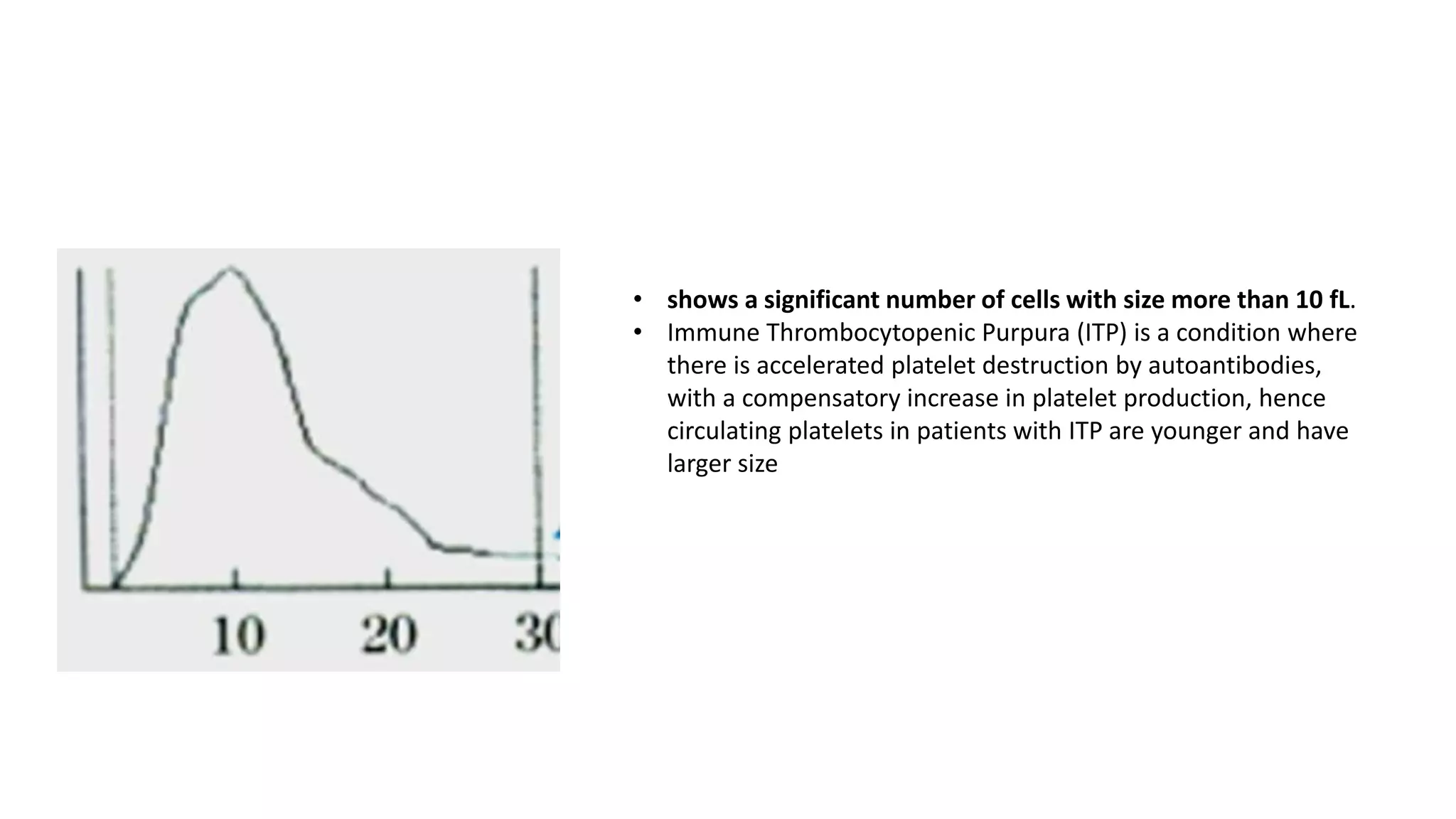
![• MPV is calculated by the formula,
• MPV (fL) = ([PCT (%)/platelet count (×10^9/l)]) × 10^5 .](https://image.slidesharecdn.com/histogram-200229021741/75/cbc-Histogram-50-2048.jpg)