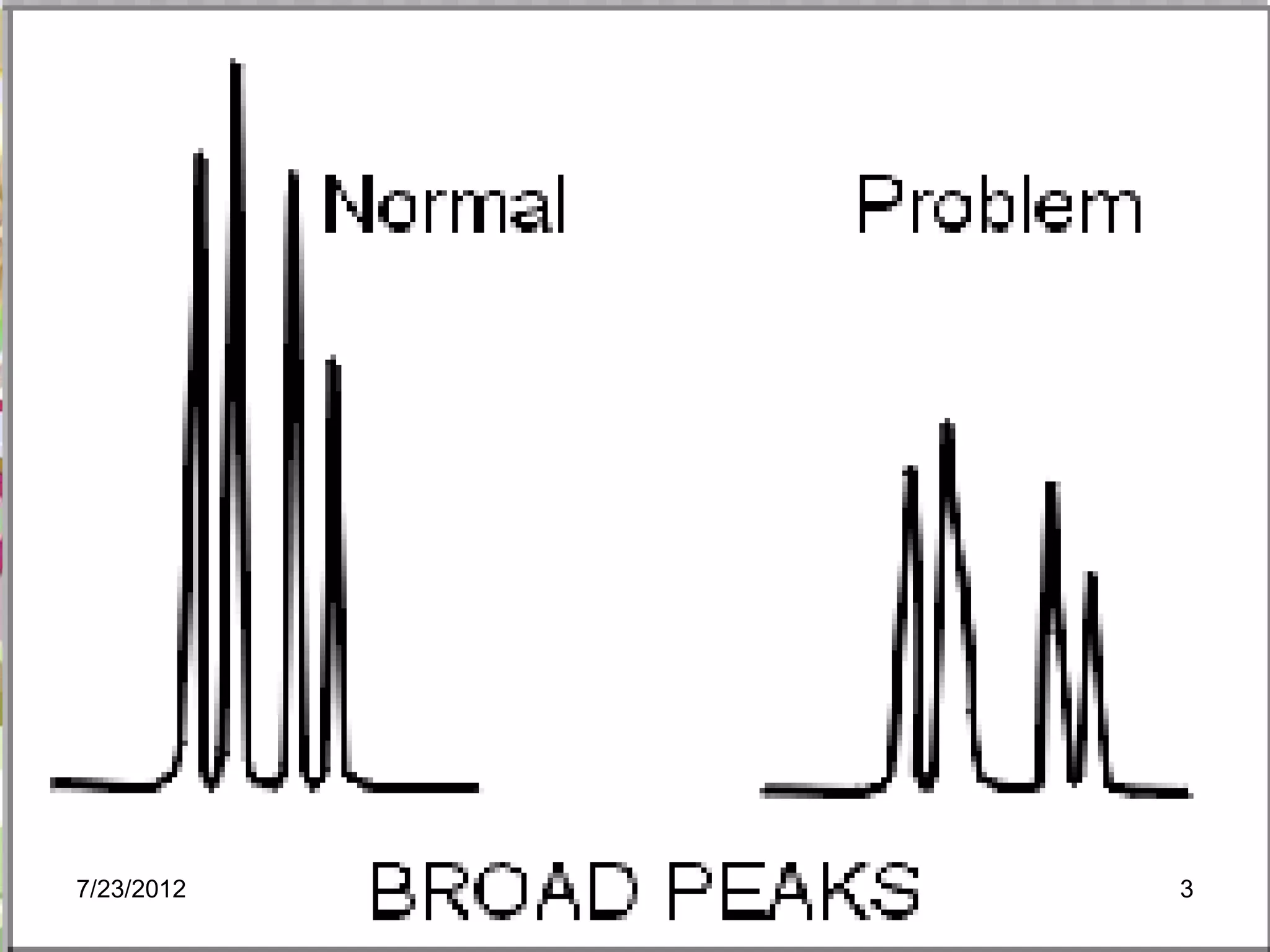
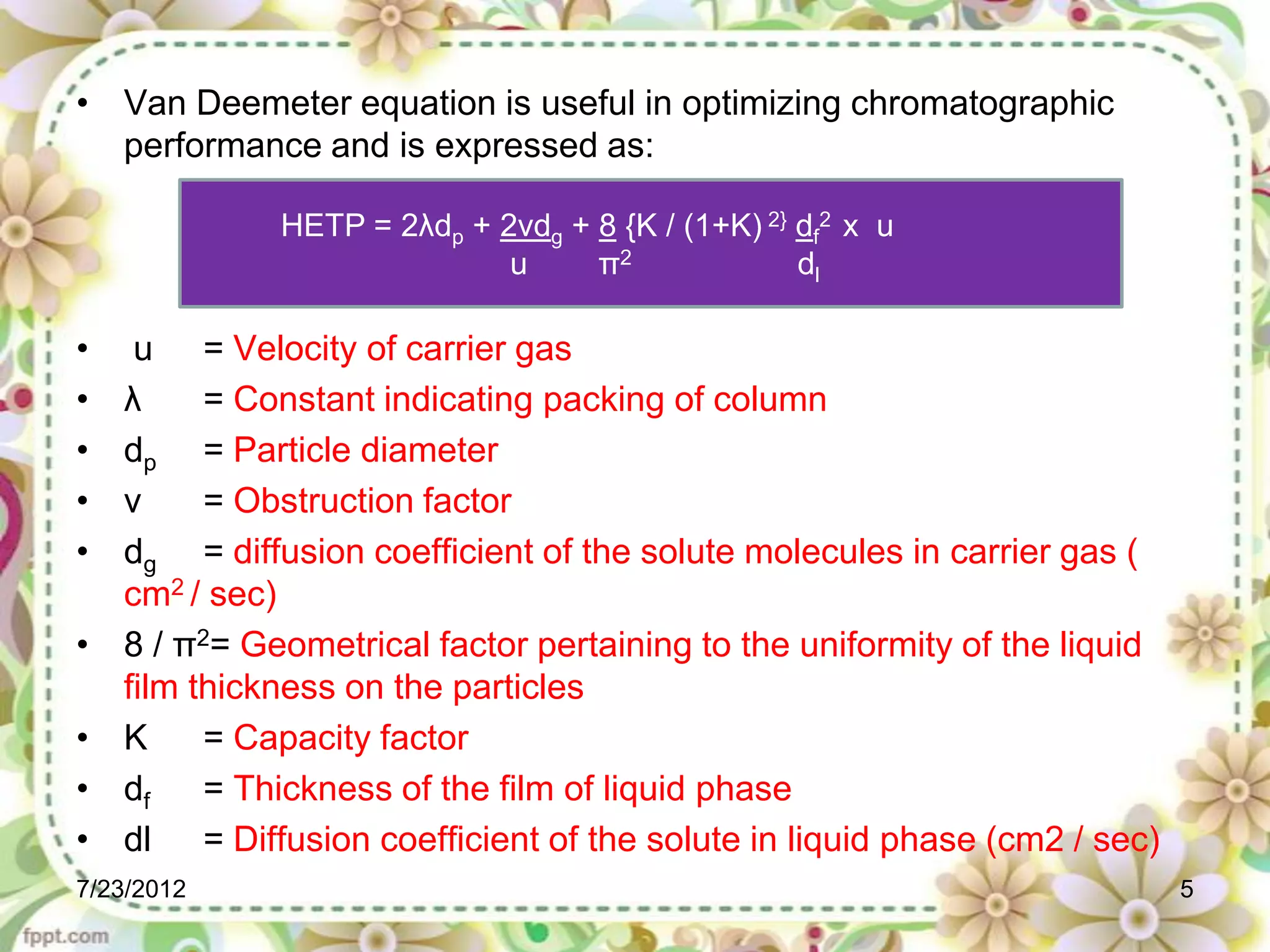
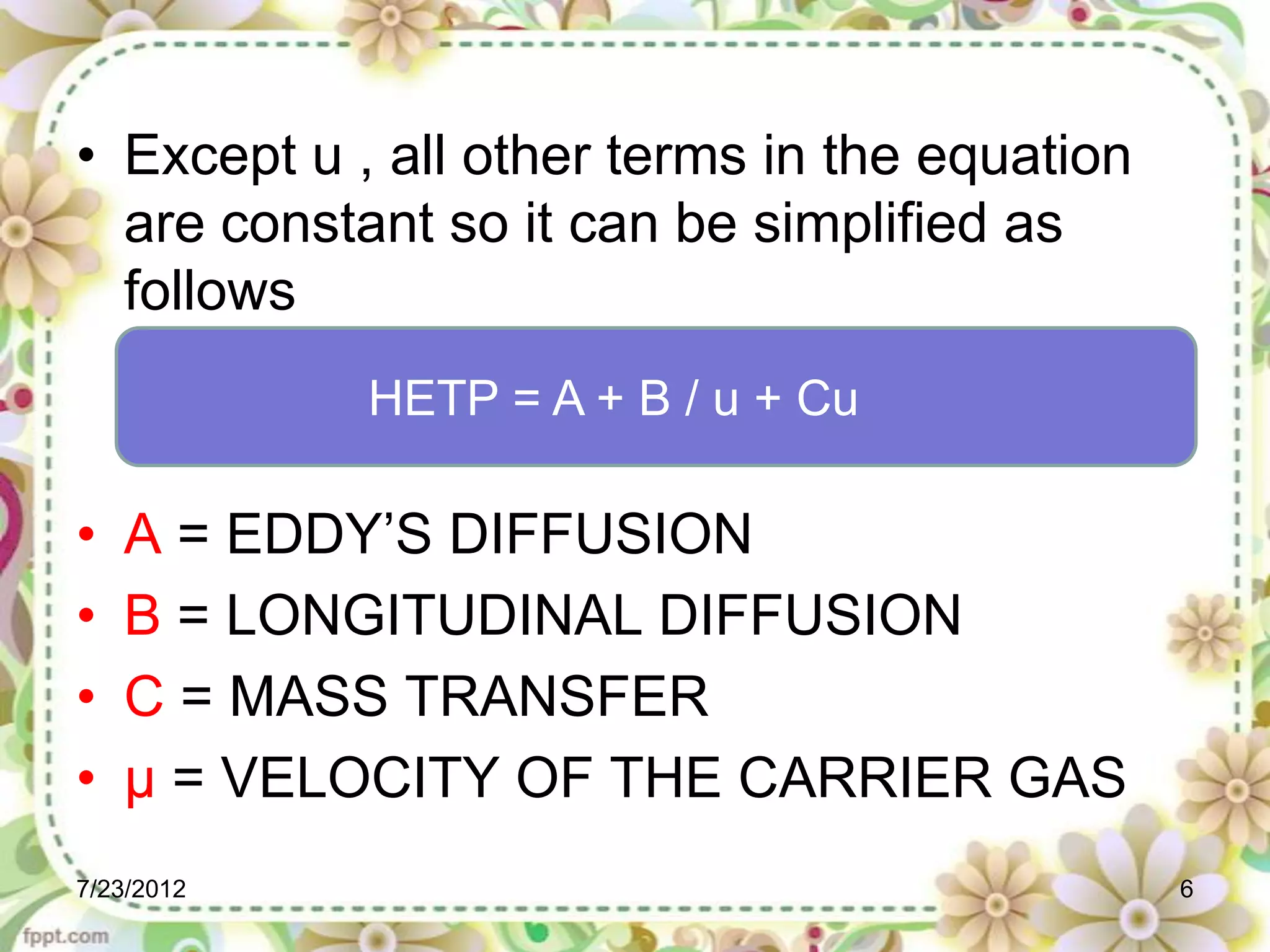
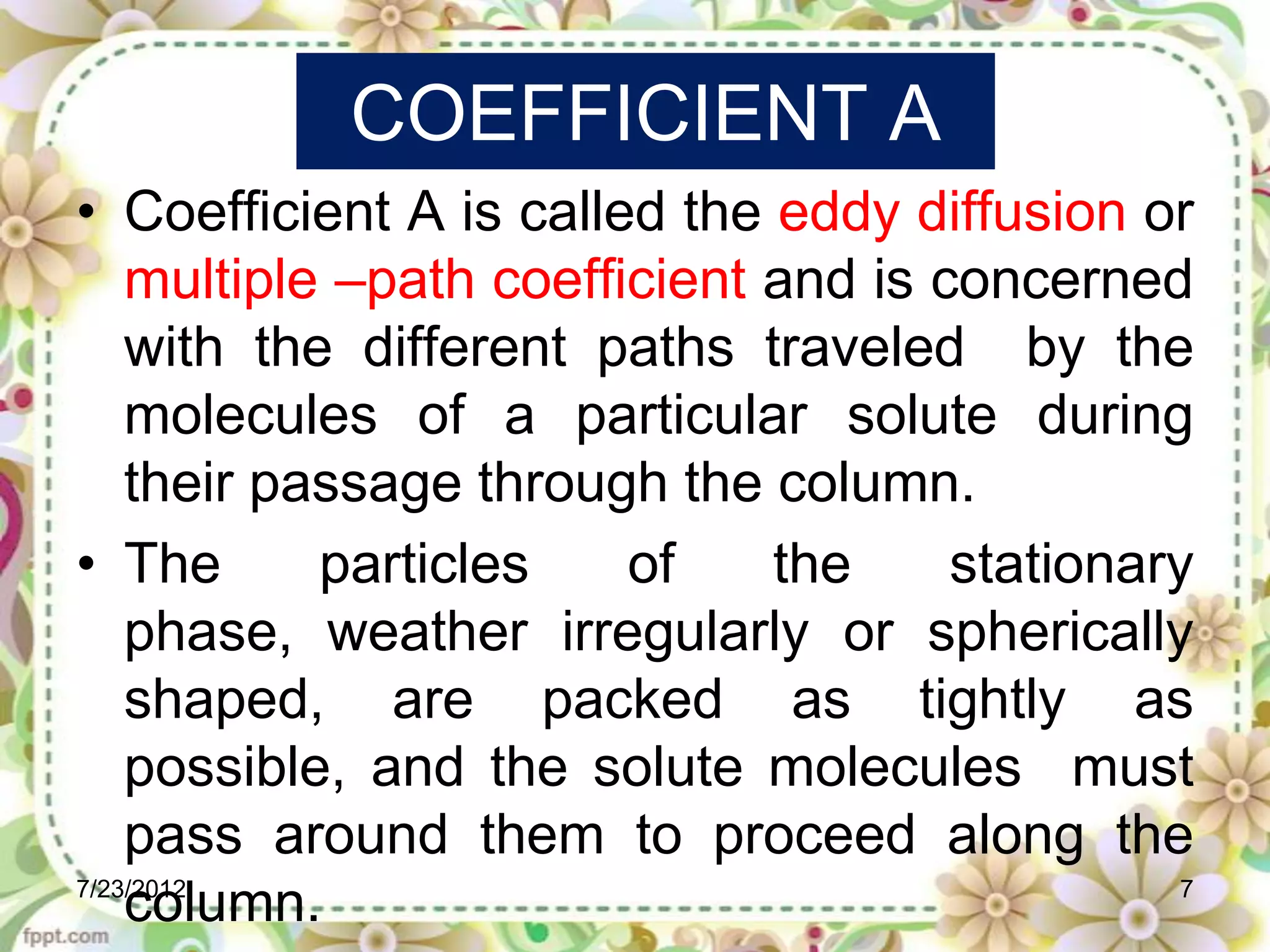
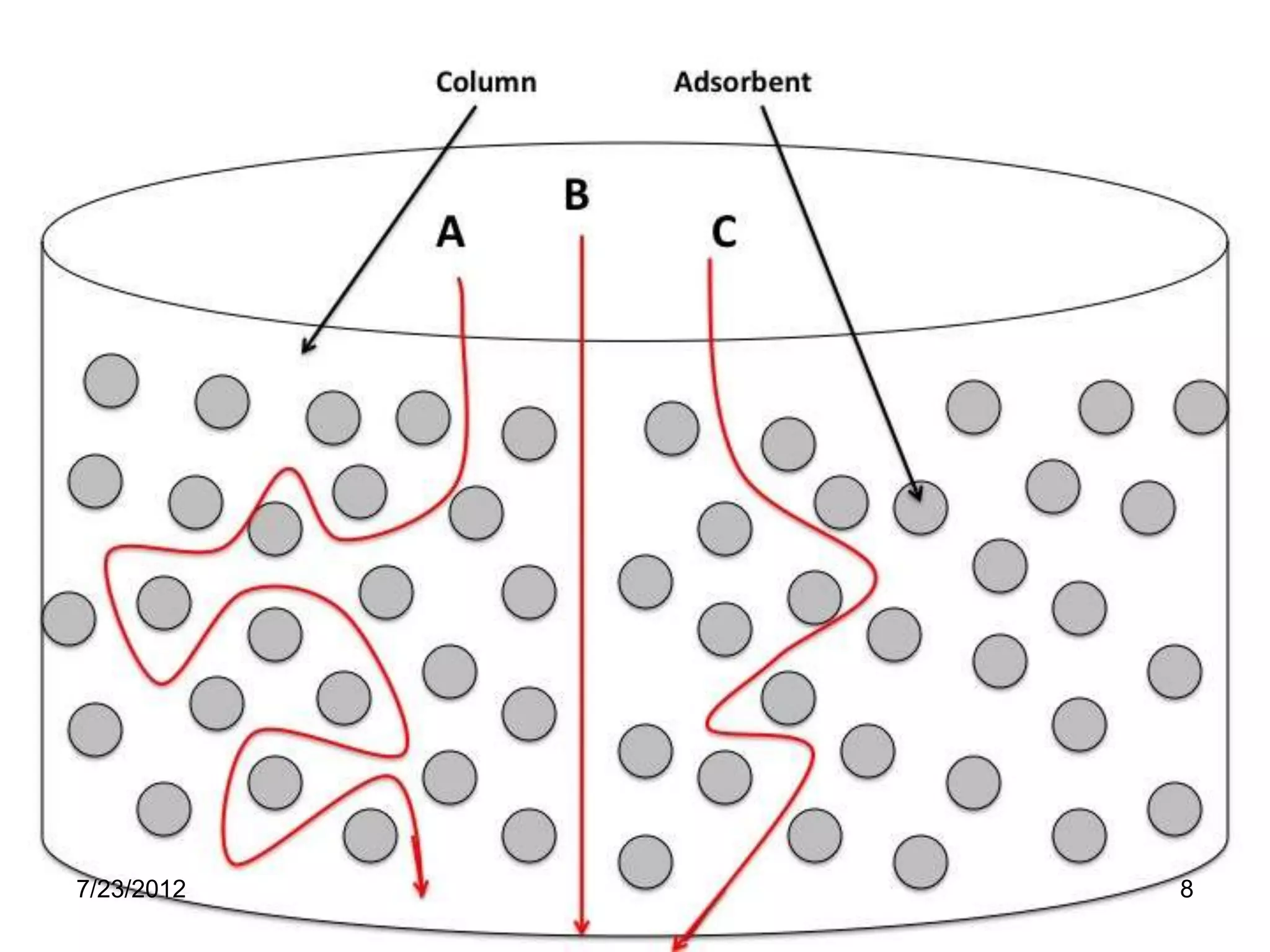
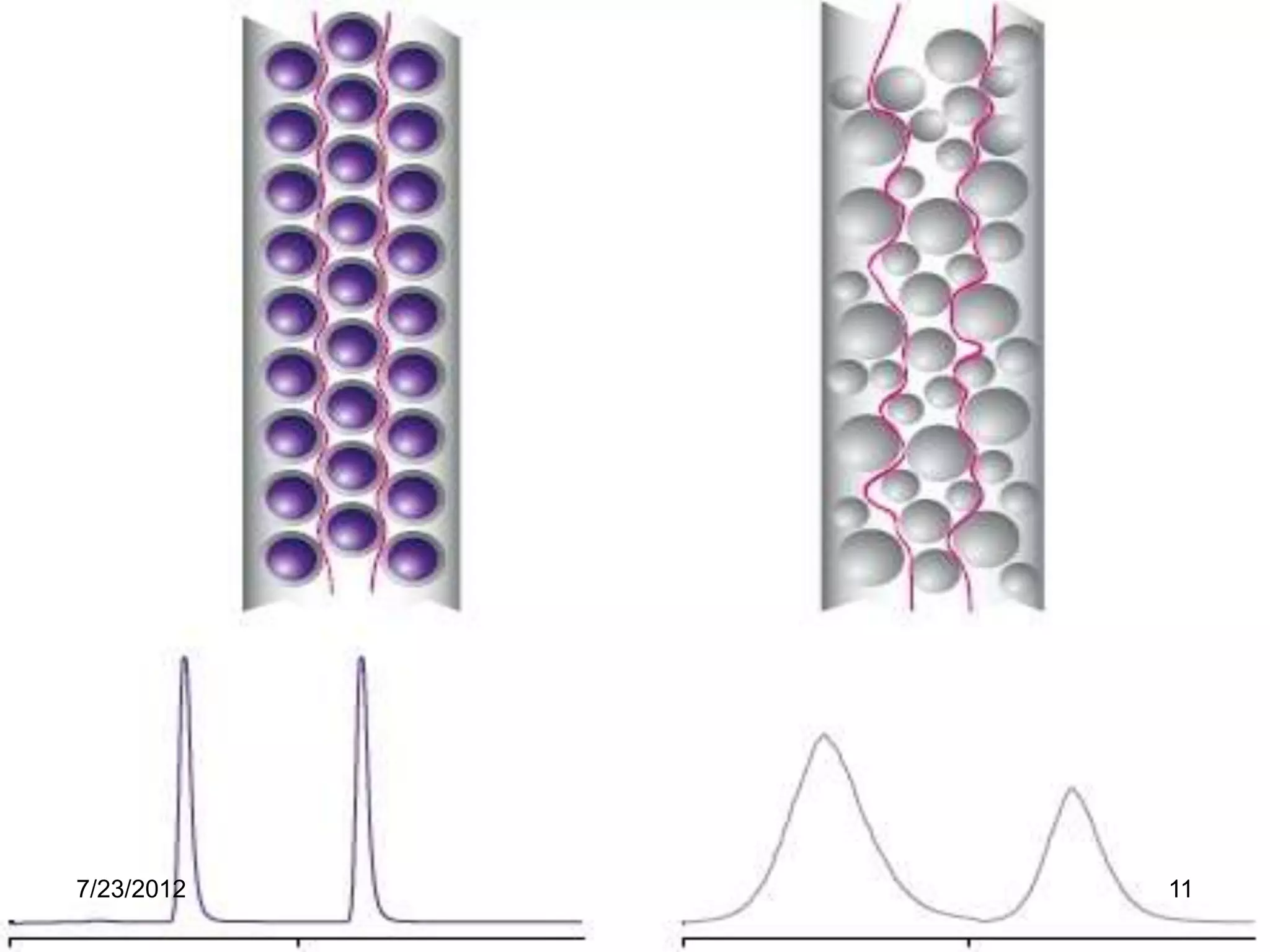
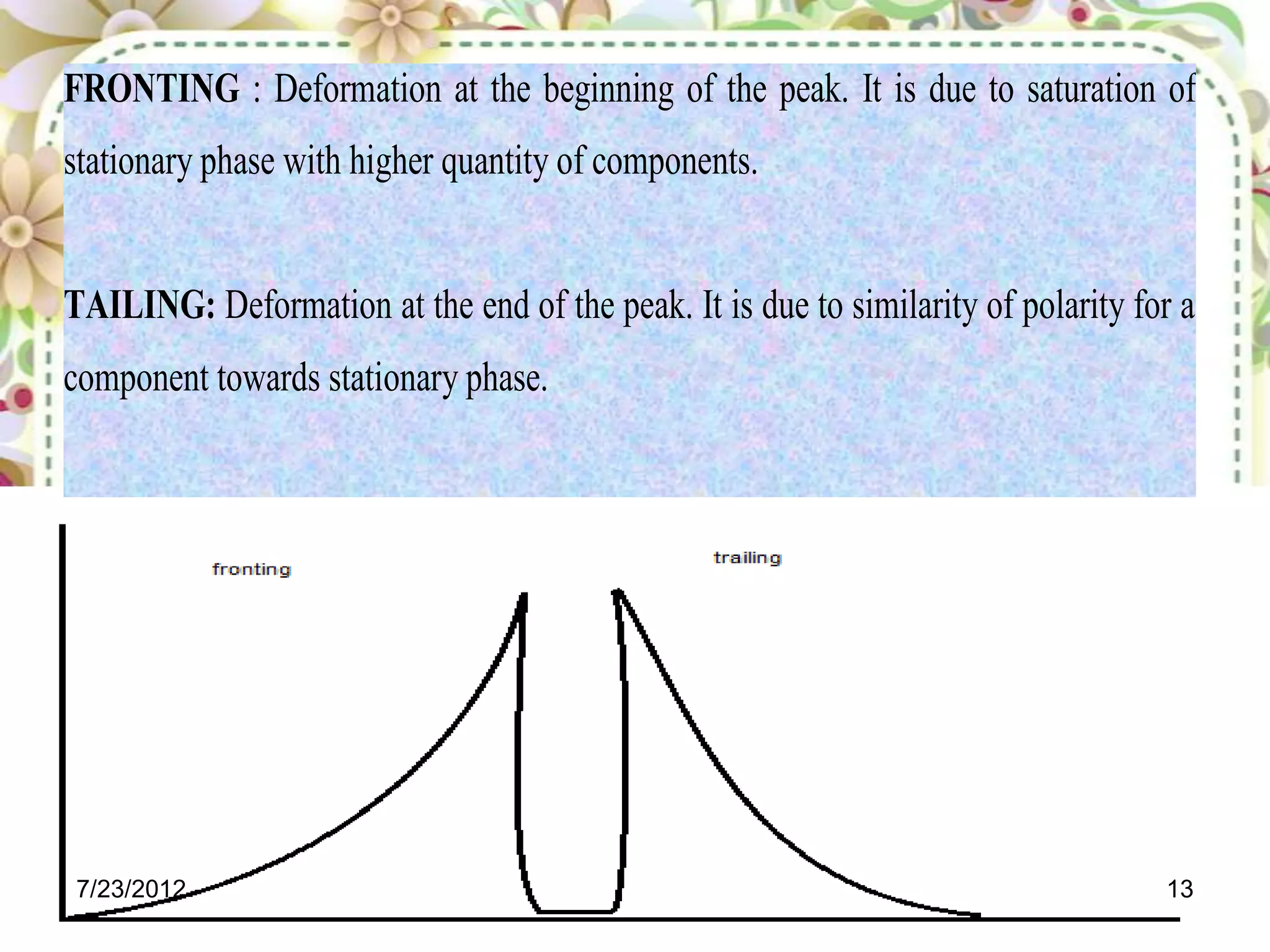
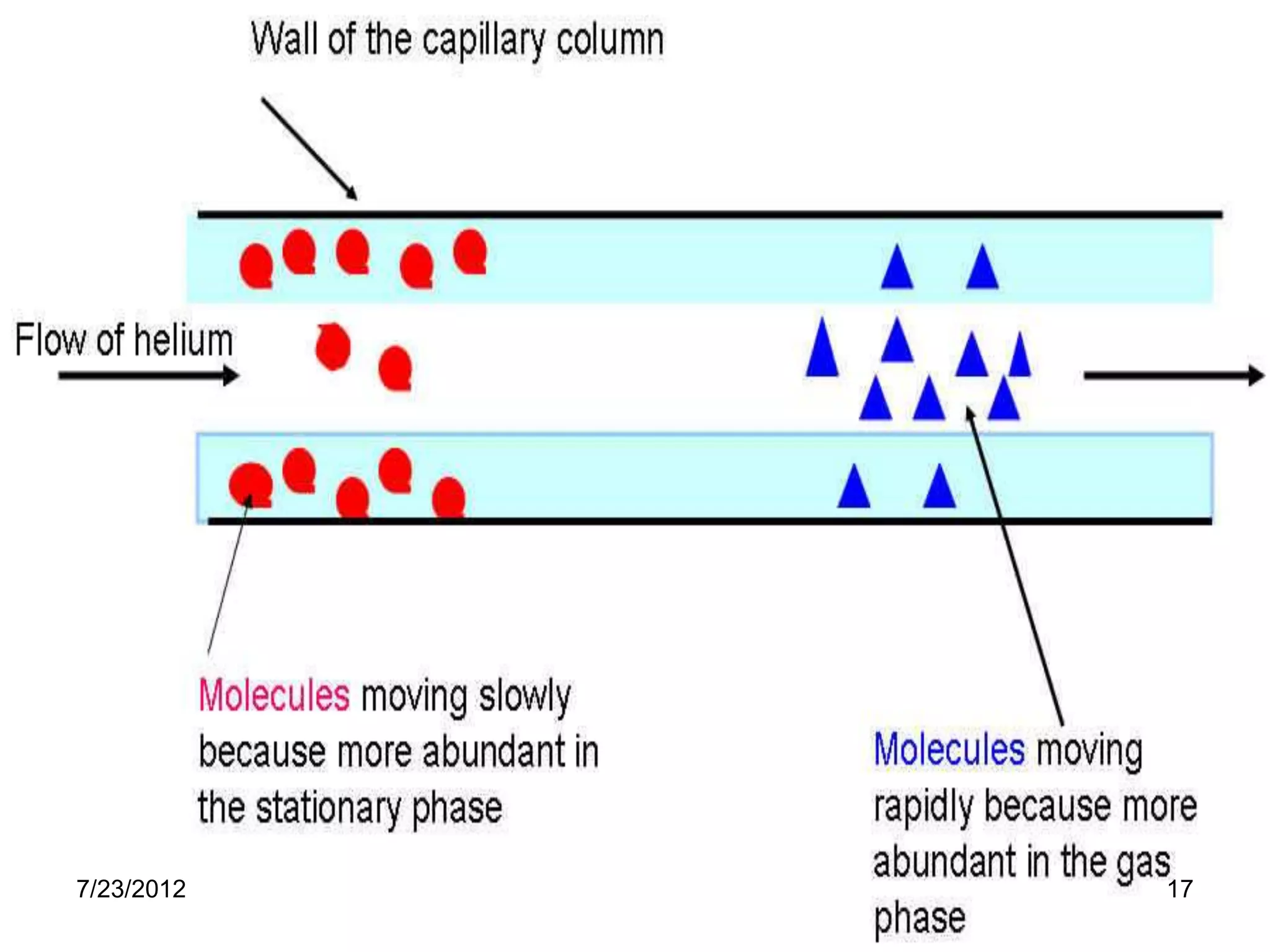
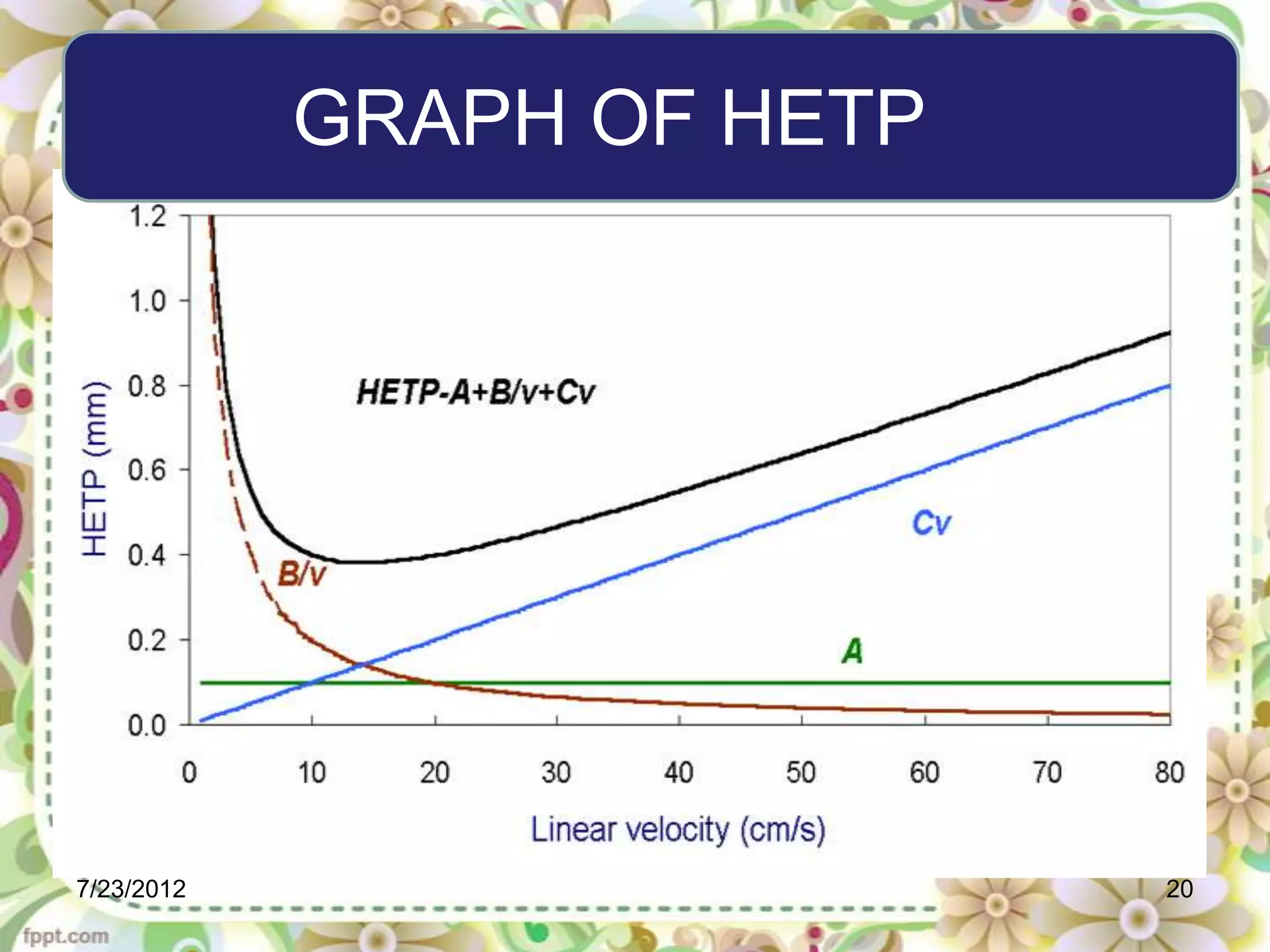
The document discusses the Van Deemter equation, which relates the height equivalent to a theoretical plate (HETP) in gas chromatography to experimental parameters like particle diameter, diffusion coefficients, and flow rate. It explains that HETP is affected by eddy diffusion, longitudinal diffusion, and mass transfer between phases. The Van Deemter equation can be used to optimize chromatographic performance by identifying conditions that minimize band broadening like adjusting flow rates and using smaller stationary phase particles.