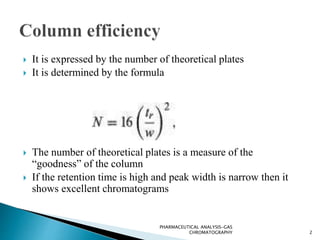
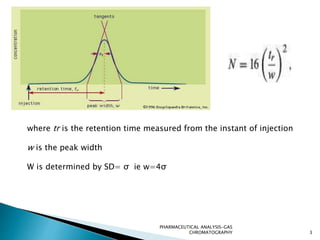
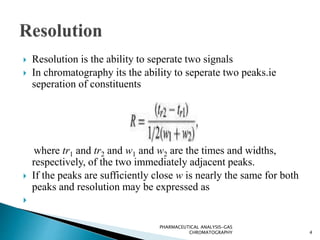
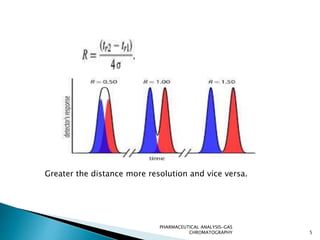
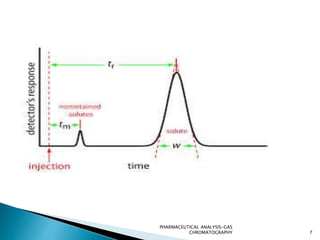
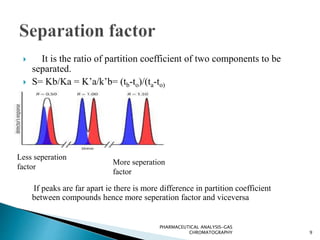
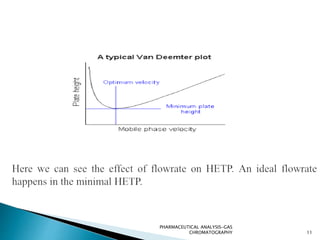
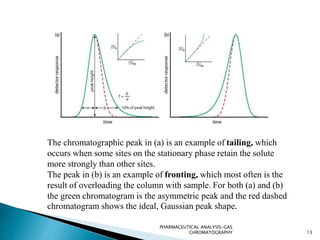
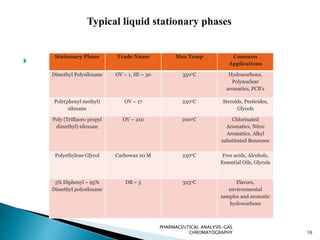
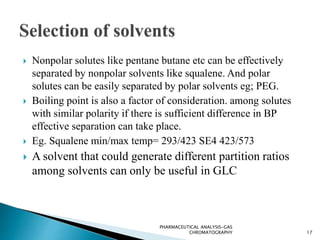
The document discusses key concepts in gas chromatography including theoretical plates, resolution, retention time, retention volume, separation factor, height equivalent to a theoretical plate (HETP), peak asymmetry, stationary phases, and considerations for choosing stationary phases. It provides definitions and equations for these terms and concepts. Examples of common stationary phase materials and their applications are also presented.