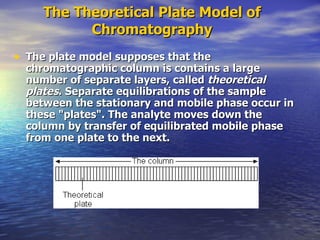
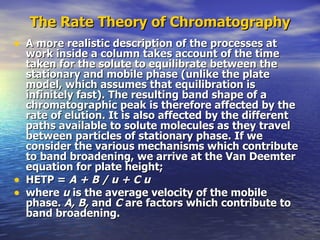
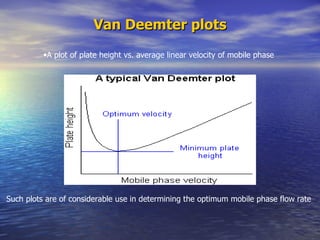
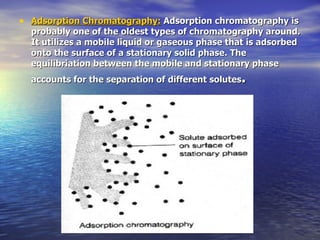
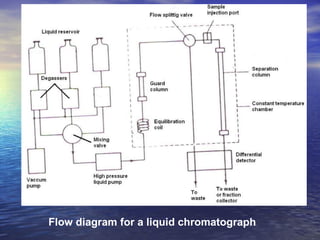
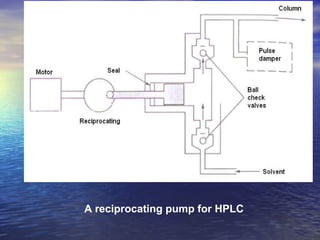
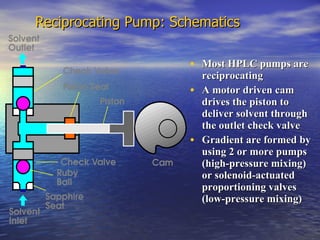
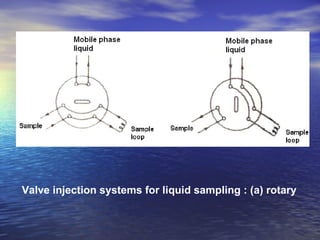
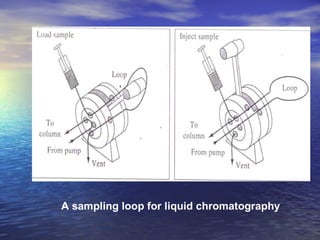
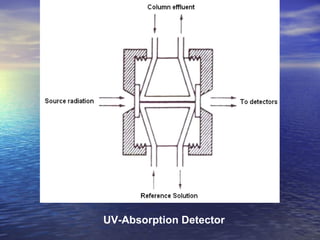
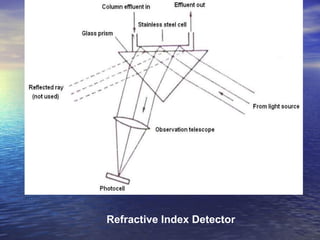
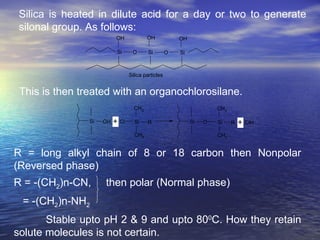
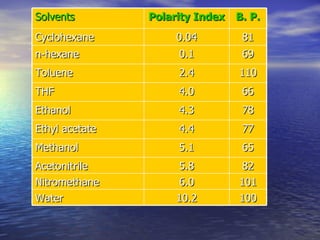
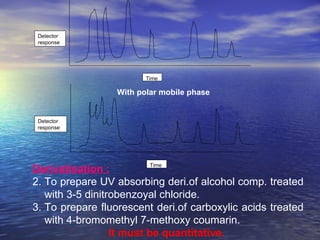
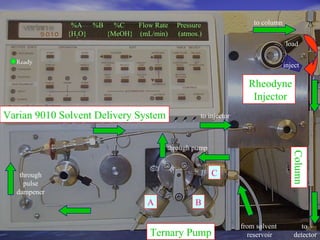
This document provides a basic introduction to chromatography, including definitions of key terms and descriptions of basic chromatography instrumentation and concepts. It discusses the basic components and functions of a liquid chromatograph, including the eluent delivery system, pumps, injection systems, columns, detectors, and common types of chromatography like adsorption and partition chromatography. It also provides explanations of fundamental chromatography concepts like retention, theoretical plates, rate theory, the van Deemter equation, and resolution.