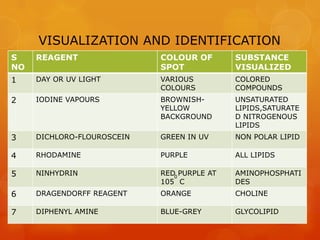
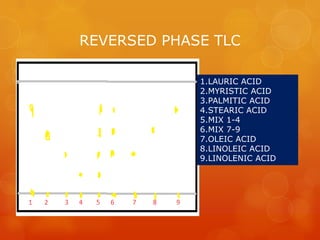
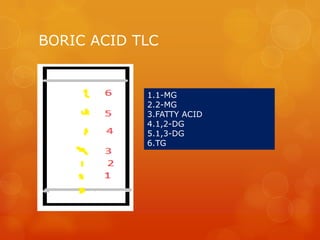
Thin layer chromatography (TLC) is a simple, inexpensive technique used to separate and identify components in organic compounds. In TLC, compounds are distributed between a stationary phase, like silica gel, and a mobile phase, like a solvent or solvent mixture. As the mobile phase travels up the plate, different components travel at different rates depending on their solubility, allowing separation. Common adsorbents and solvent systems used for lipid separation are described. Various detection reagents can be used to visualize separated components under UV light or by producing colored spots. Examples are given of separations achieved for different lipid classes and subclasses using TLC.