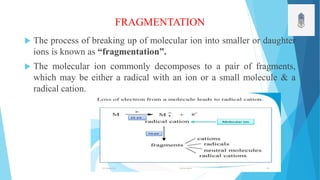
This document discusses fragmentation techniques in mass spectroscopy. It begins by defining mass spectroscopy as a technique used to determine molecular weight of compounds. The key points made are:
1) Molecular ions formed during ionization undergo fragmentation into smaller daughter ions.
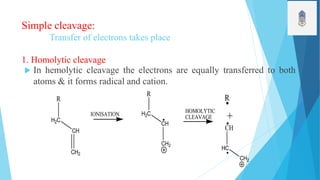
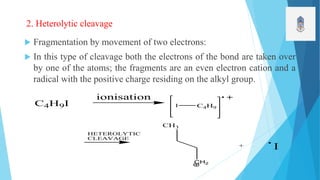
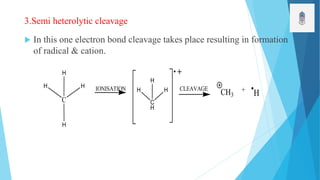
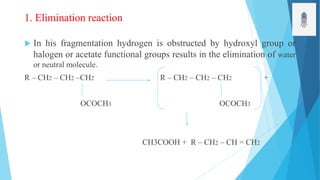
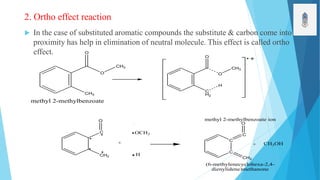
2) Fragmentation occurs through simple cleavage like homolytic, heterolytic, or semi-heterolytic cleavage or through rearrangement reactions like elimination, ortho, or McLafferty rearrangements.
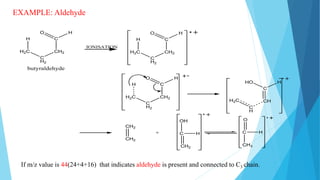
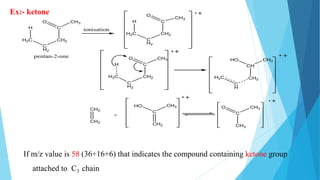
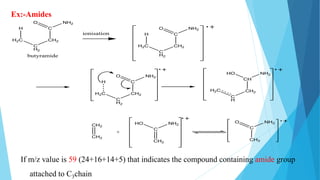
3) Specific fragmentation patterns can provide information about functional groups present like aldehydes producing m/z 44 ions or ketones producing m/z 58 ions.