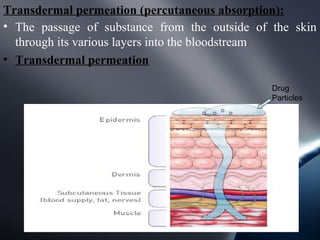
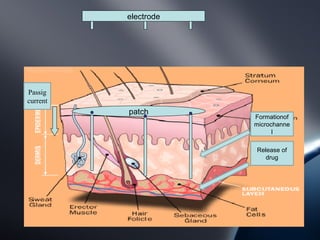
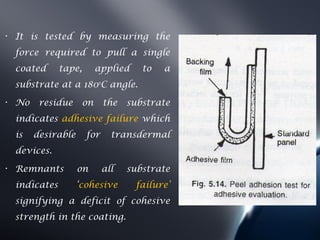
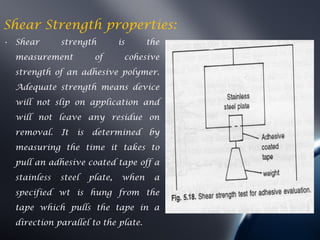
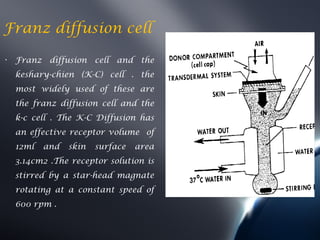
Transdermal drug delivery systems (TDDS) deliver drugs through the skin and into systemic circulation at a controlled rate. TDDS provide advantages like avoidance of first-pass metabolism and allowing controlled drug levels. The skin is a barrier, so permeation involves partitioning into the stratum corneum then diffusion across layers. Factors like a drug's physicochemical properties, the delivery system composition, and skin conditions influence permeation kinetics. TDDS have components like polymer matrices, drugs, and permeation enhancers. They are evaluated for properties such as adhesive peel adhesion to ensure removal does not damage skin.