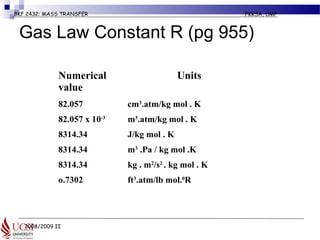
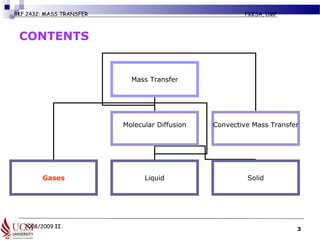
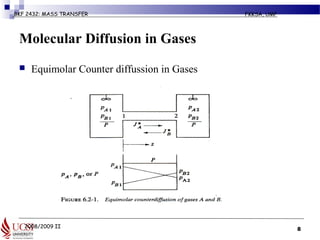
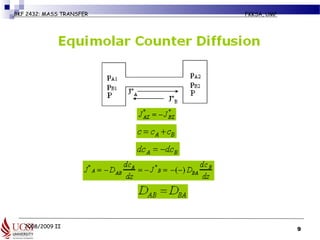
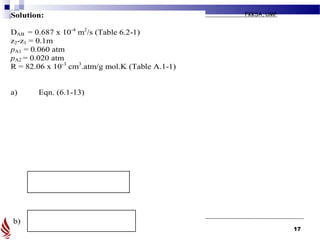
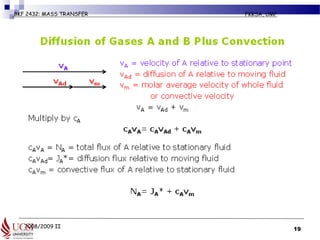
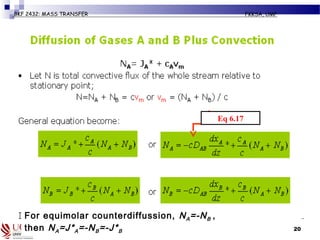
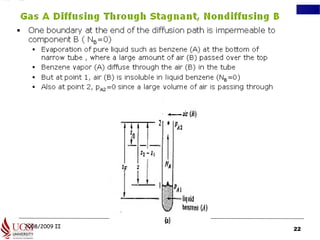
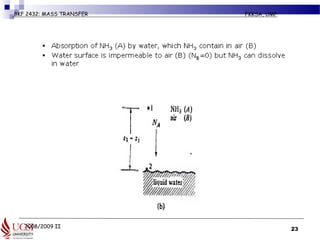
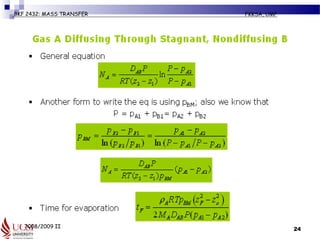
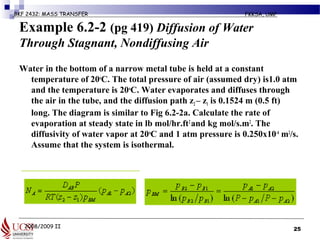
The document discusses principles of molecular diffusion in gases. It covers topics such as equimolar counter diffusion, diffusion through cross-sectional areas like spheres, and calculating diffusion coefficients. Examples and problems are provided to demonstrate how to calculate flux and diffusion rates in various scenarios, including diffusion between binary gas mixtures and evaporation from surfaces. Methods for estimating gas diffusivity are also presented.













![2008/2009 II
BKF 2432: MASS TRANSFER FKKSA, UMP
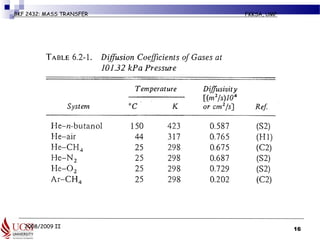
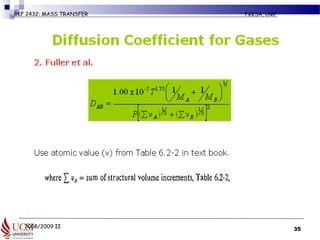
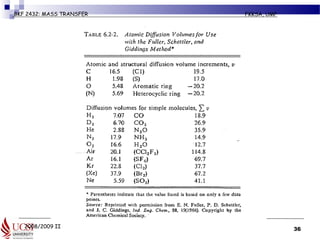
Example 6.2-5 (pg 427) Estimation of
Diffusivity of a Gas Mixture
• Normal butanol (A) is diffusing through air (B) at 1 atm abs. Using
the Fuller et al. method, estimate the diffusivity DAB for the following
temperatures and compare with the experimental data.
• Given MA (butanol) = 74.1 kg (mass)/kg mol,
• MB (air) = 29 kg (mass)/kg mol]
a) For 0o
C.
b) For 25.9o
C
c) For 0o
C and 2.0 atm abs
37](https://image.slidesharecdn.com/chap1a-moleculardiffusioningas2-150419121756-conversion-gate01/85/Chap-1-a-molecular-diffusion_in_gas-2-37-320.jpg)