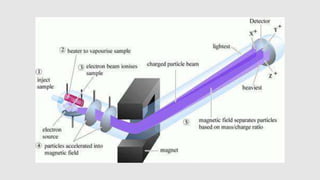
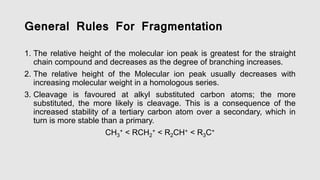
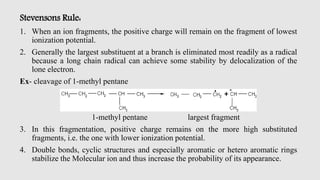
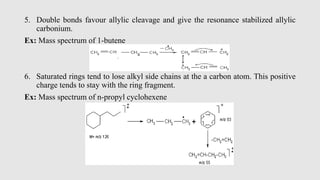
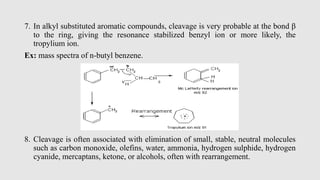
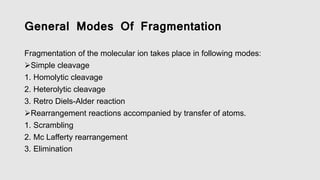
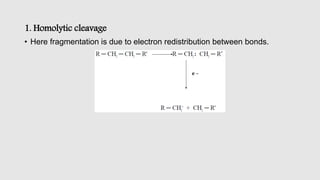
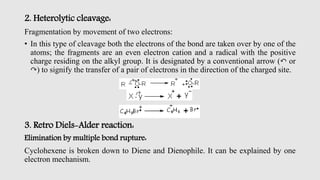
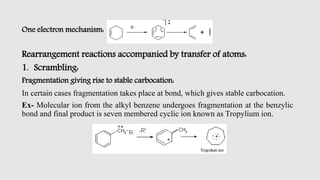
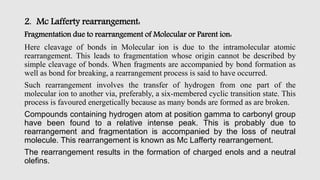
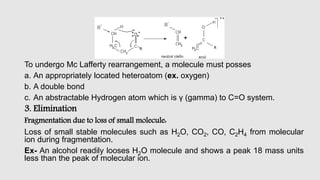
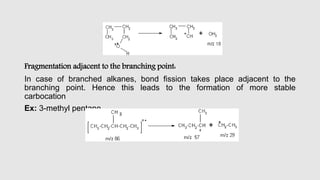
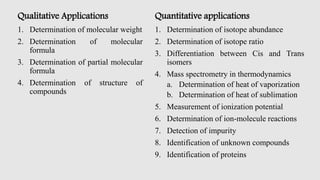
Mass spectrometry is an instrumental technique used to provide qualitative and quantitative information by ionizing molecules and separating them based on their mass-to-charge ratio. The document details the process of mass fragmentation, the rules governing fragmentation, and different modes of fragmentation including homolytic and heterolytic cleavage, as well as rearrangement reactions. It also outlines applications of mass spectrometry in various fields, including structure elucidation, detection of impurities, and quantitative analysis.