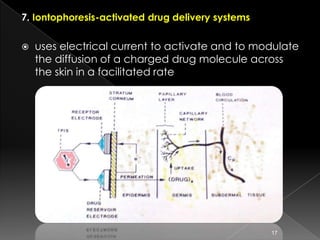
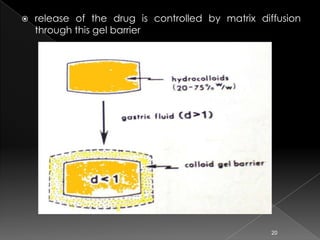
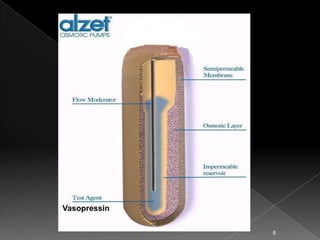
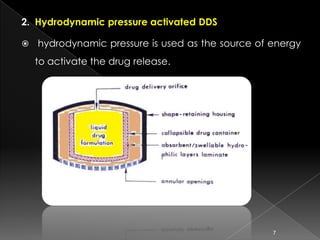
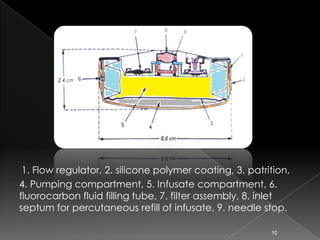
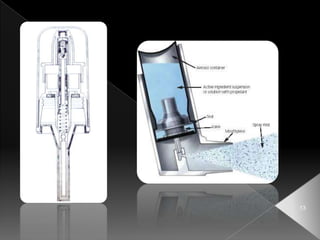
This document discusses different types of activation modulated drug delivery systems (DDS). It describes DDS that are activated by physical, chemical, or biological means. Some examples of physically activated DDS include osmotic pressure-activated, hydrodynamic pressure-activated, vapour pressure-activated, and mechanically activated systems. Magnetically activated and sonophorosis activated DDS are also mentioned. The document provides details on the mechanisms and equations for rate of drug release for some of these systems. It further discusses iontophoresis-activated and hydration-activated DDS and provides one example for each.








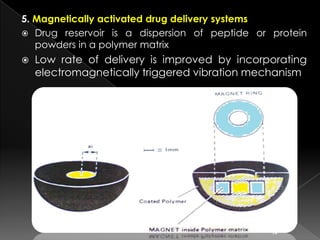
![ Coating polymer can be a ethylene-vinyl acetate
copolymer or silicon elastomers.
These systems have been used to deliver protein drugs,
such as bovine serum albumin
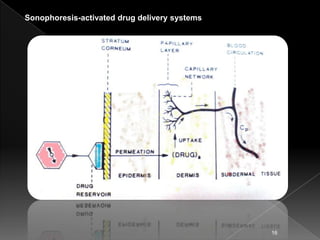
6. Sonophoresis-activated drug delivery systems
Utilize ultrasonic energy to activate the delivery of the
drugs from a polymeric drug delivery device
can be fabricated from either a non degradable
polymer, such as ethylene-vinyl acetate copolymer,
a bio erodible polymer such as poly[bis(p-
carboxyphenoxy)alkane anhydride].
15](https://image.slidesharecdn.com/activation-130318041328-phpapp01/85/Activation-modulated-drug-delivery-systems-15-320.jpg)