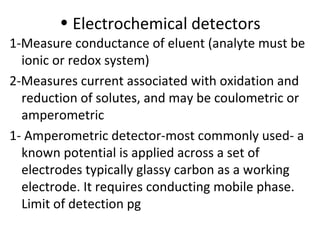
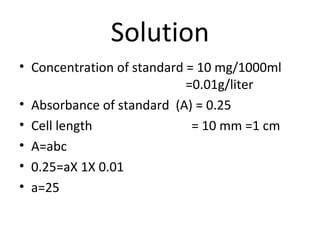
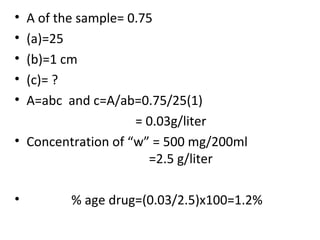
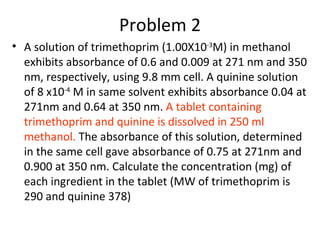
Okay, let me break this down step-by-step:
* We are given absorbance values for known concentrations of trimethoprim and quinine standards at two wavelengths
* Using Beer's law, we can calculate the molar absorptivities (ε) of each at each wavelength
* Then using the absorbance readings of the combined sample, and the ε values, we can calculate the concentrations of each in the sample
* From the concentrations and sample volume, the masses and thus amounts of each in the tablet can be determined
Does this help explain the approach? Let me know if you would like me to show the full working.