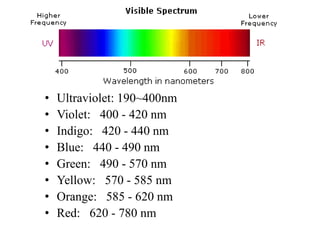
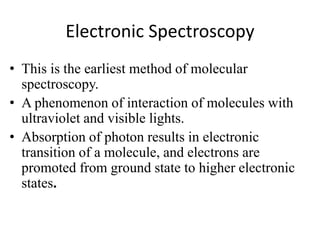
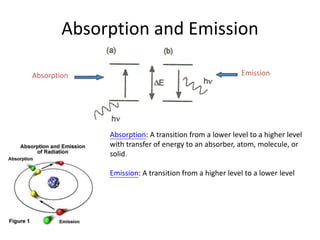
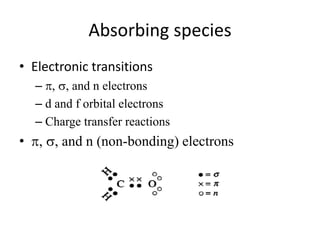
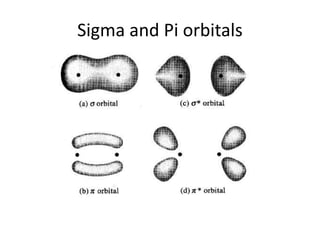
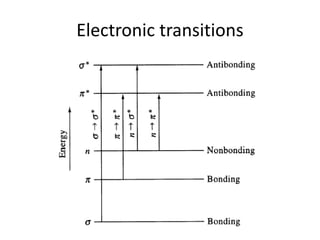
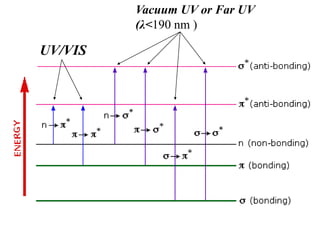
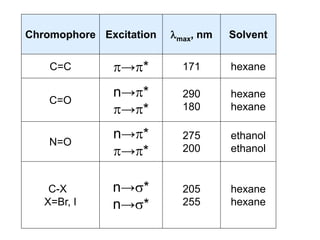
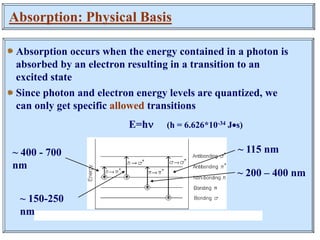
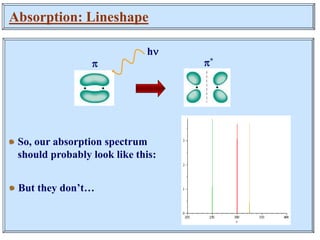
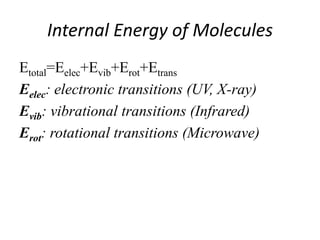
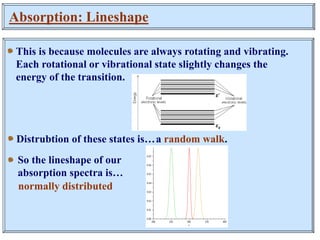
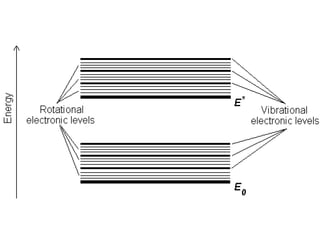
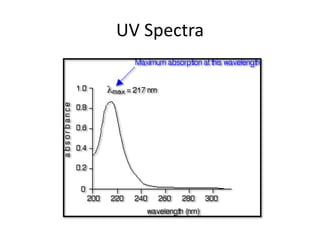
This document discusses UV-Vis spectroscopy. It begins by defining the ultraviolet and visible wavelength ranges from 190-780 nm. It then explains that UV-Vis spectroscopy involves electronic transitions of molecules when exposed to these wavelengths, promoting electrons from ground states to excited states. The document discusses terms like chromophores, auxochromes, and bathochromic/hypsochromic shifts related to UV absorptions. It also describes different types of electronic transitions that can be detected by UV-Vis spectroscopy, including σ→σ*, n→σ*, n→p*, and p→p* transitions involving different orbital types.