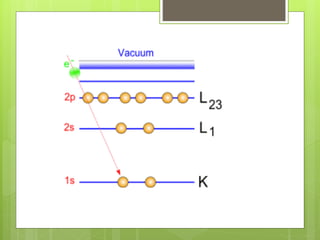
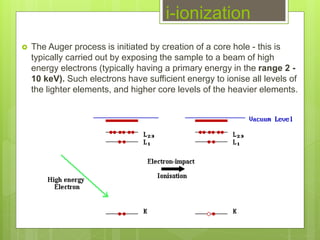
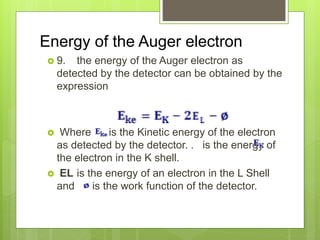
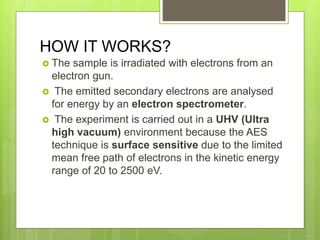
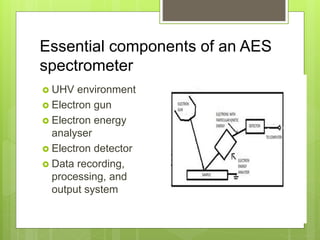
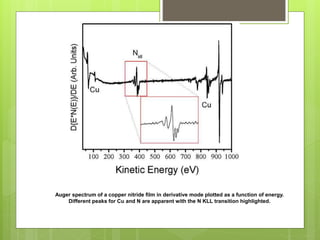
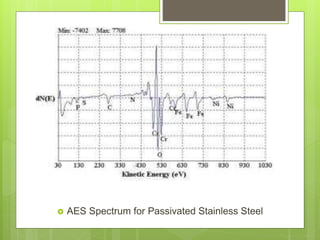
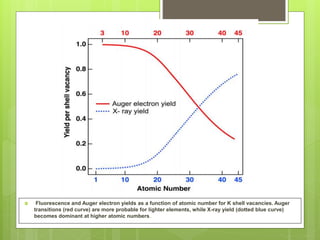
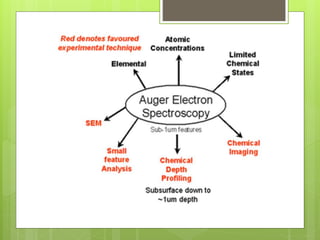
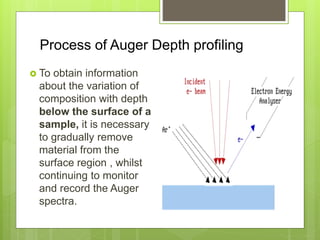
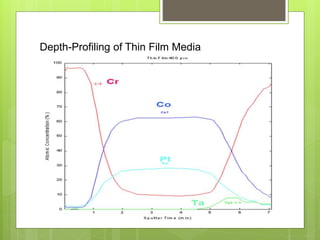
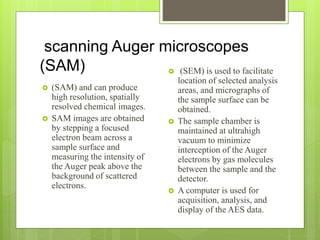
Auger electron spectroscopy (AES) is an analytical technique used to analyze the surface chemistry of materials. It works by (1) removing a core electron from the sample using a high-energy electron beam, (2) causing an electron to fill the resulting vacancy and emit an Auger electron, and (3) analyzing the kinetic energy of the emitted Auger electrons to determine the elemental composition of the top 1-10 nanometers of the sample surface. AES can also be used to create depth profiles by combining it with argon ion sputtering to sequentially remove layers from the surface. Typical applications of AES include analyzing thin film layers, surface oxides, and corrosion processes.