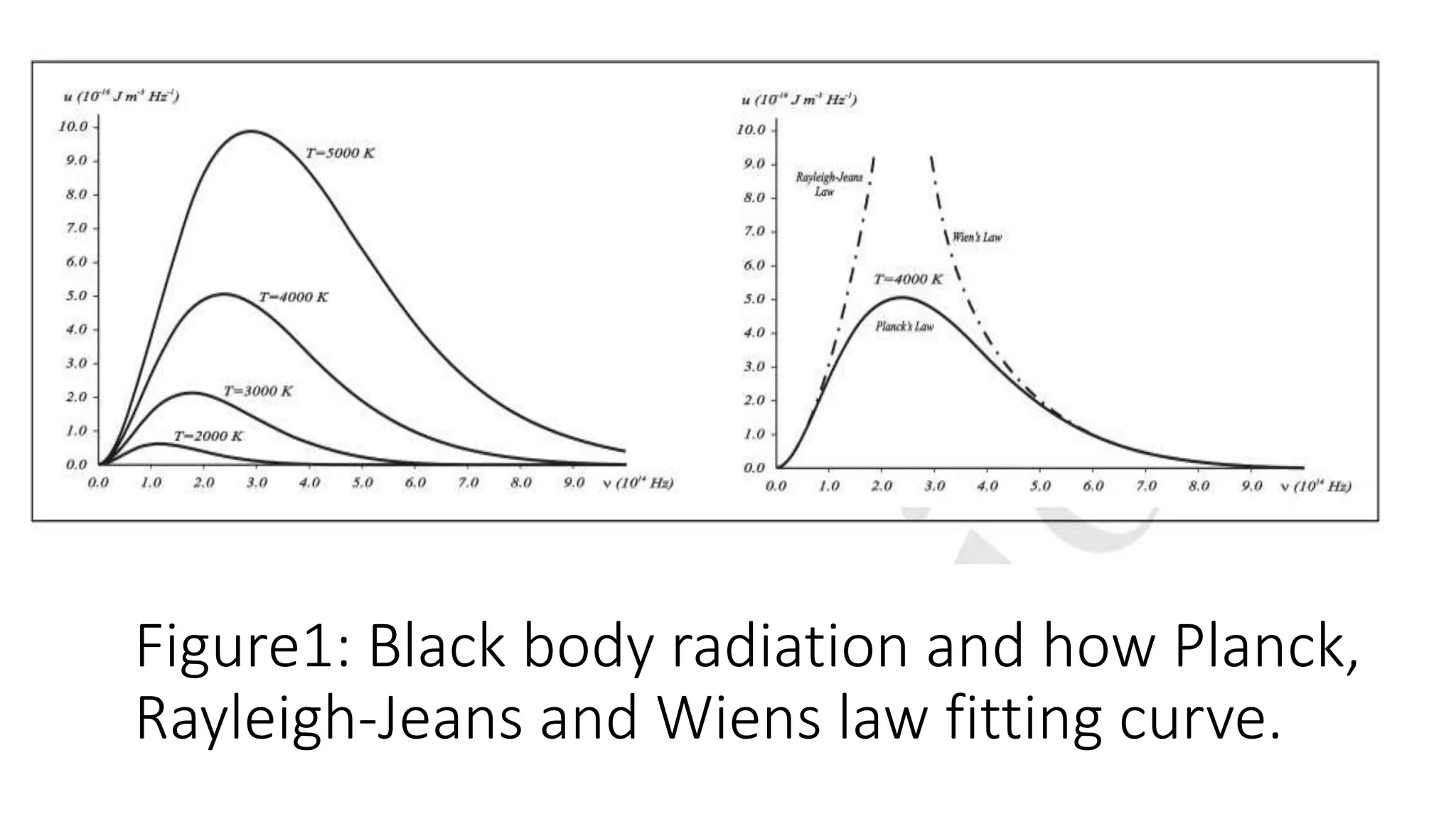
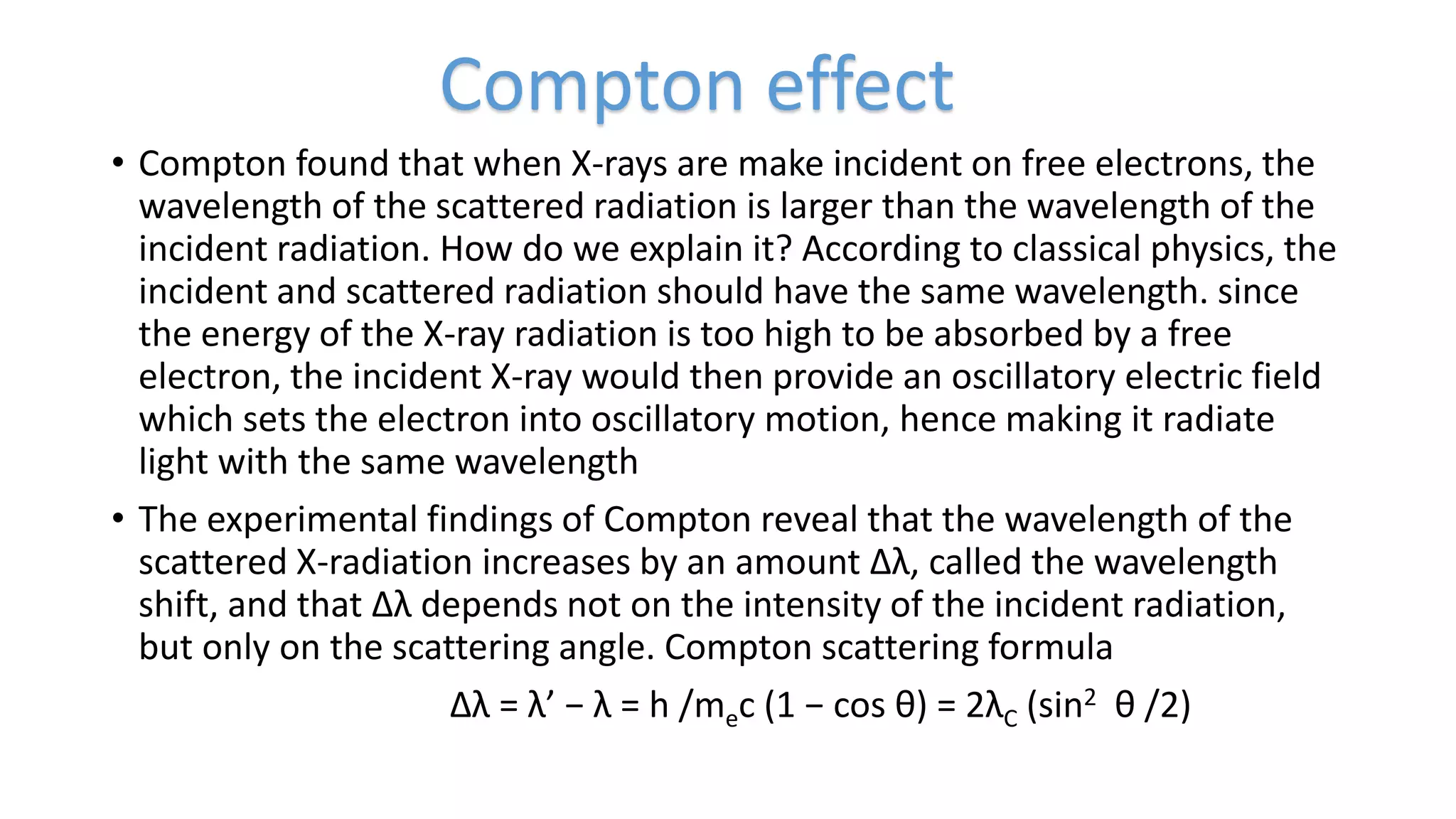
The document discusses the wave-particle duality of matter and radiation, highlighting key experiments and phenomena such as black body radiation, the photoelectric effect, and the Compton effect. It emphasizes that particles like electrons exhibit both wave-like and particle-like properties, challenging classical physics concepts. The document also details experiments such as the Davisson-Germer and double-slit experiments which demonstrate the interference patterns associated with particles.