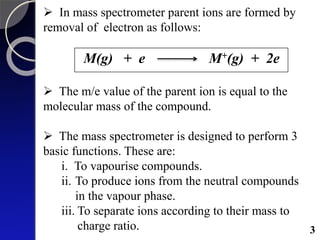
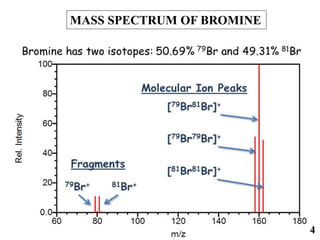
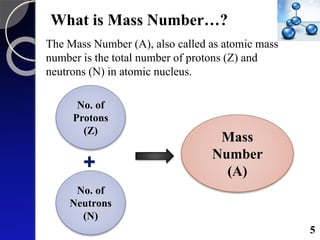
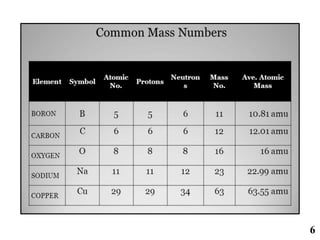
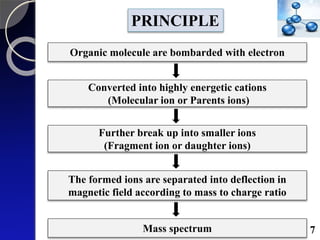
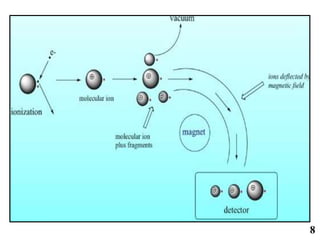
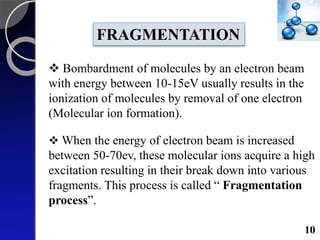
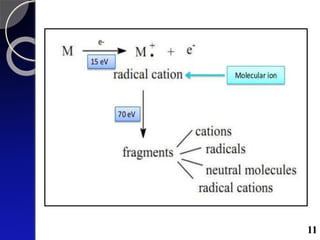
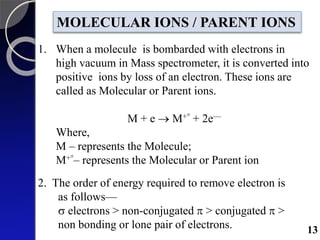
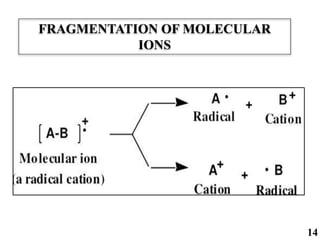
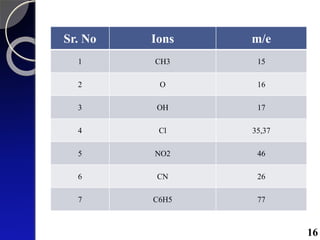
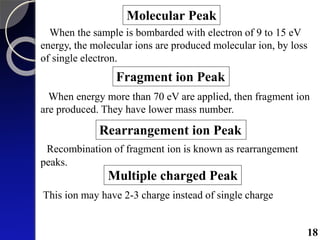
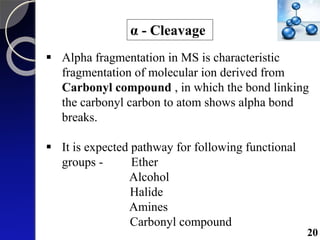
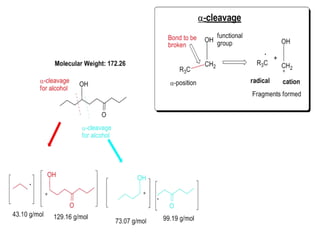
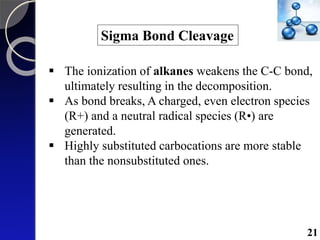
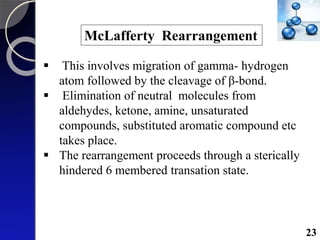
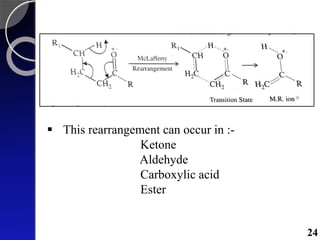
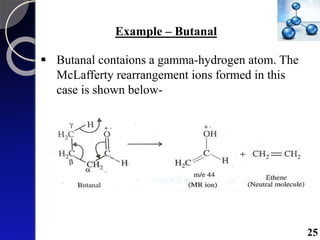
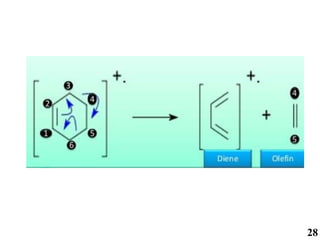
The document discusses mass spectrometry (MS), an analytical technique that converts samples into positive ions to measure their mass-to-charge ratios. It covers the principles of ionization, fragmentation processes, and various types of ions produced, including parent and fragment ions. The presentation highlights key fragmentation mechanisms, such as alpha cleavage and McLafferty rearrangement, along with the types of peaks observed in mass spectra.