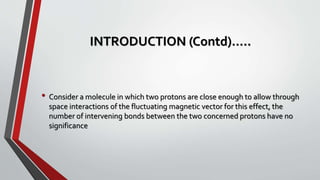
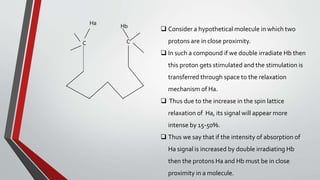
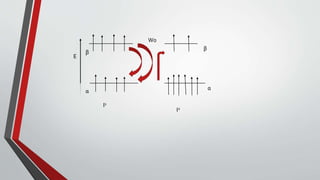
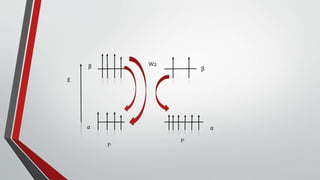
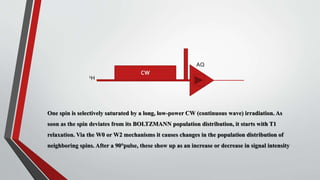
The document discusses the nuclear Overhauser effect (NOE), which occurs when two protons are in close proximity within a molecule. Irradiating one proton perturbs its spin distribution and affects the relaxation of the other nearby proton. This causes the intensity of the other proton's signal to increase or decrease, indicating their proximity. The NOE provides information about molecular geometry without requiring coupling between nuclei and can reveal which protons are near each other in a structure.