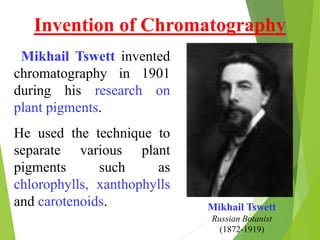
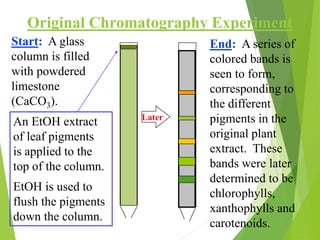
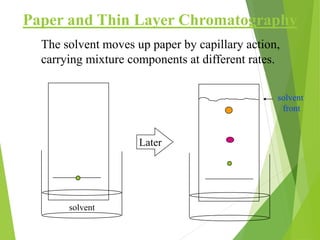
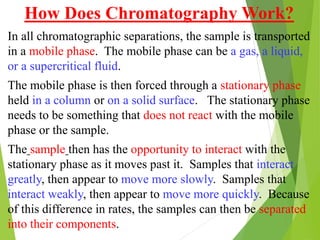
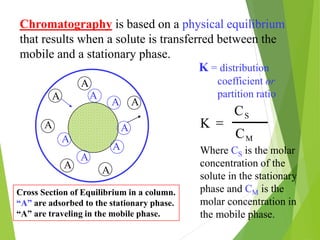
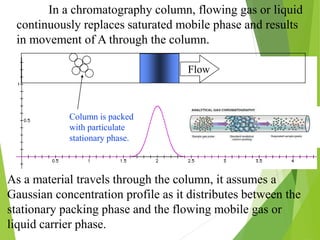
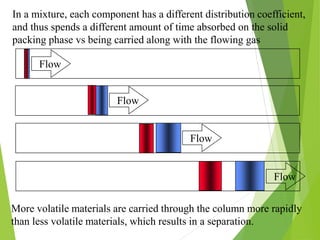
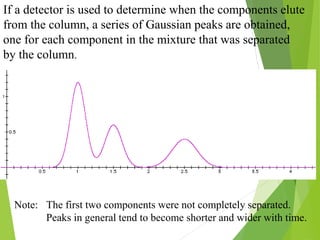
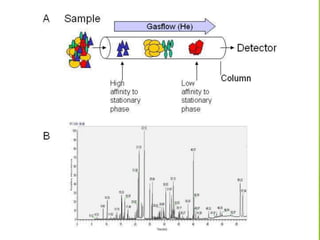
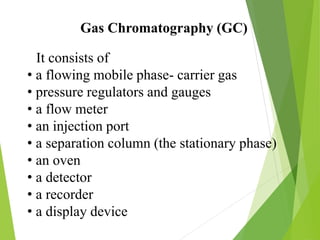
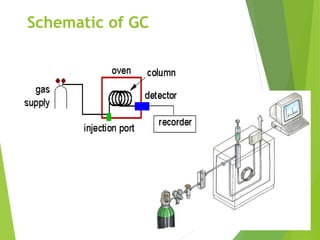
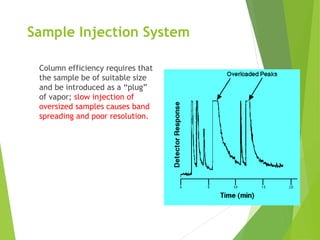
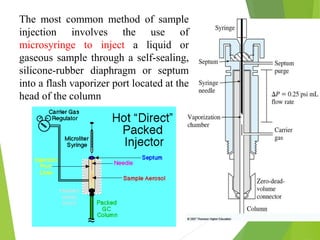
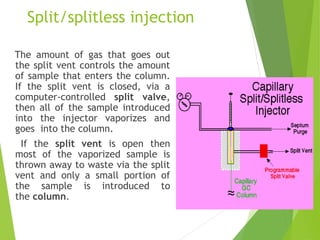
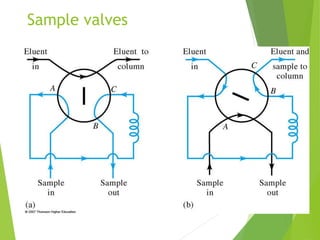
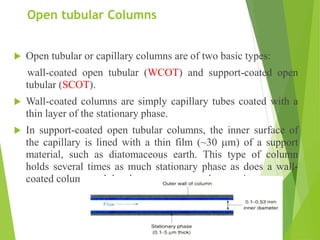
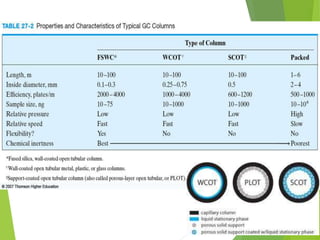
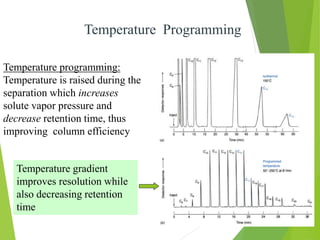
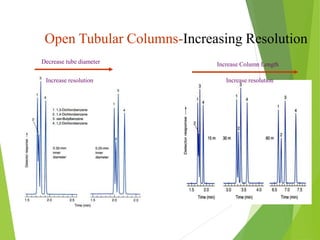
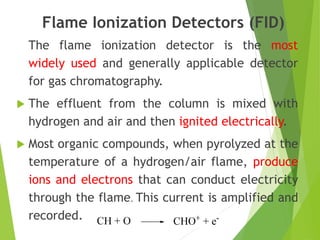
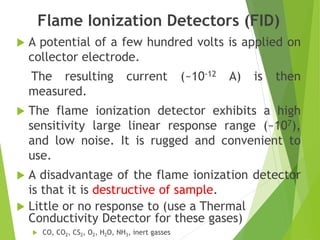
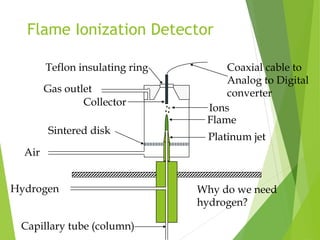
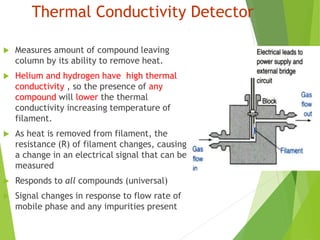
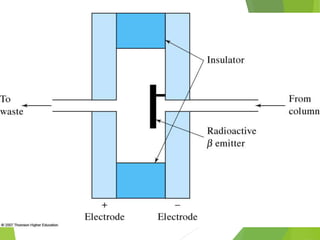
This document discusses the history and principles of gas chromatography, a technique developed by Mikhail Tswett in 1901 for separating plant pigments. It outlines the components and workings of gas chromatography, including the role of mobile and stationary phases, types of columns, and the processes involved in detection. Various detection systems and their characteristics, including flame ionization and thermal conductivity detectors, are also detailed.