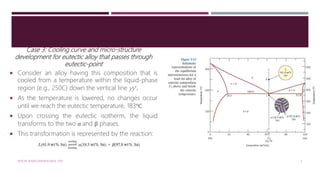
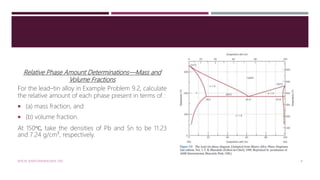
The document discusses the lead-tin (Pb-Sn) phase diagram and the microstructural development of eutectic alloys through various cooling curve scenarios. It elaborates on the solidification processes for different alloy compositions as they cool, including specific transformations when passing through solid solution and eutectic points. Additionally, it includes practice problems for determining phase compositions and fractions in various alloy scenarios.