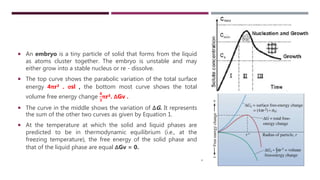
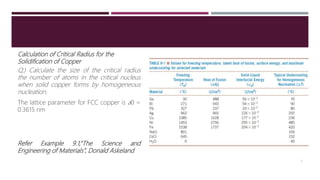
The lecture focuses on the solidification process of metals and alloys, emphasizing nucleation and growth mechanisms including homogeneous and heterogeneous nucleation, as well as planar and dendritic growth. It covers critical concepts such as critical radius for nucleation, cooling curves, and the factors affecting solidification time. By the end of the lecture, students should understand the nucleation process and differences between growth types, supported by calculations of critical radius and examples from cooling curves.