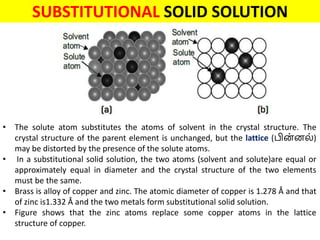
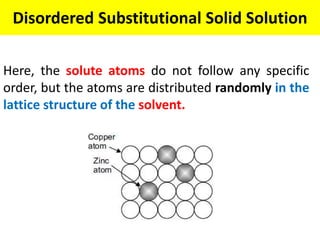
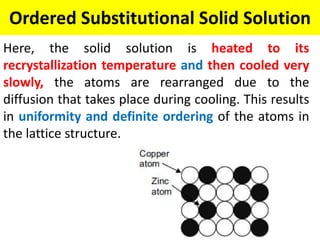
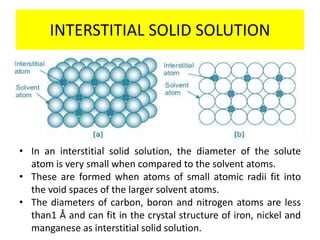
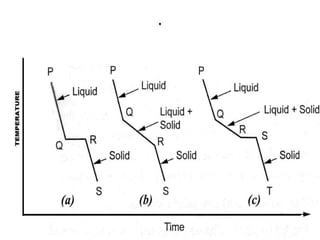
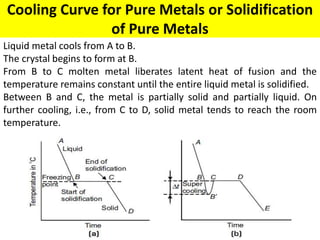
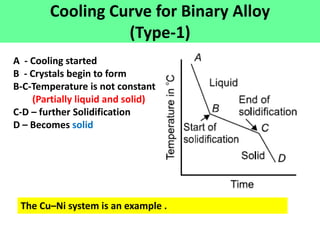
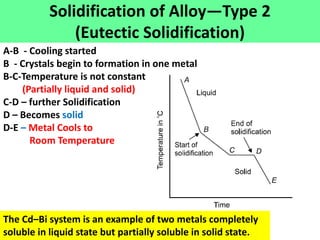
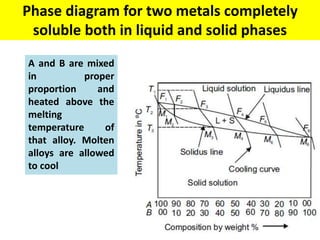
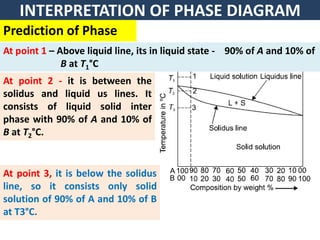
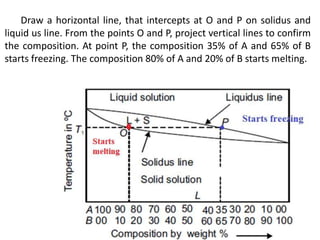
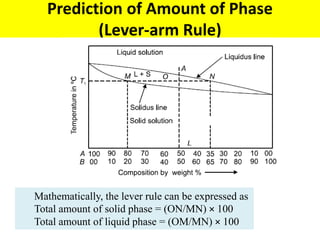
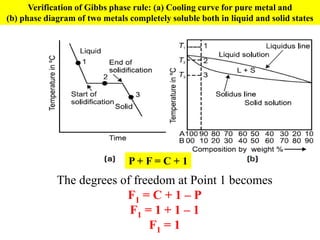
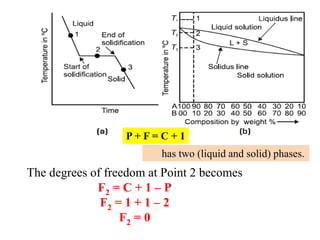
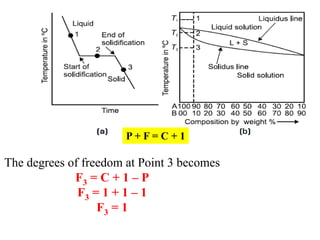
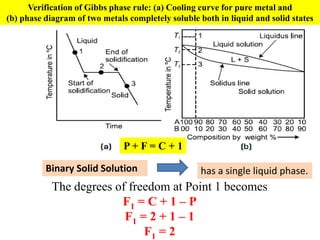
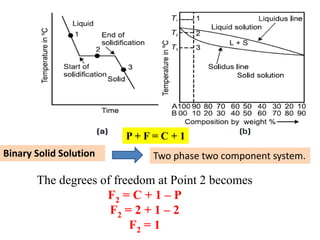
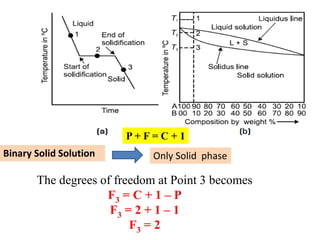
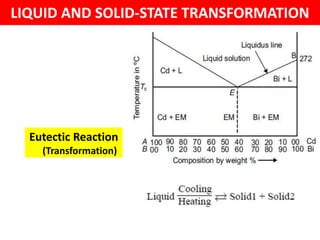
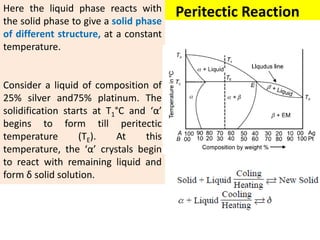
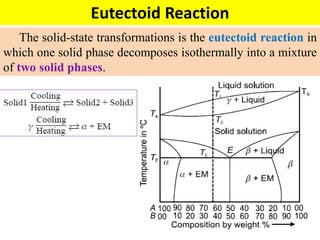
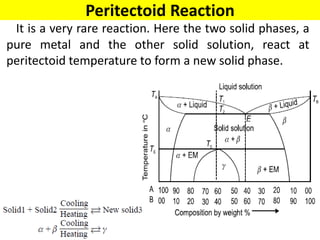
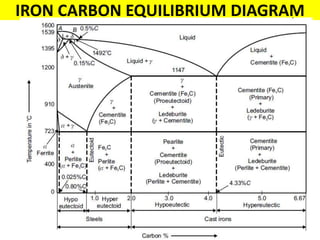
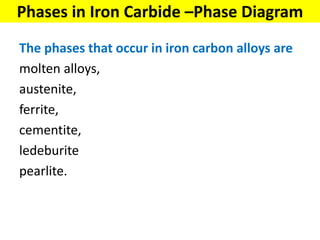
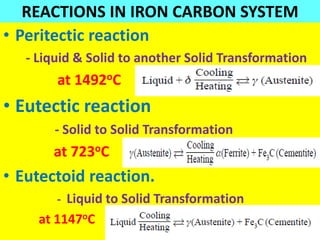
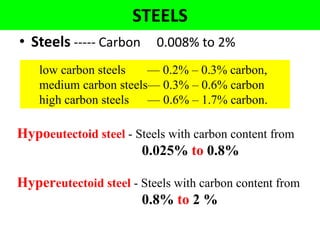
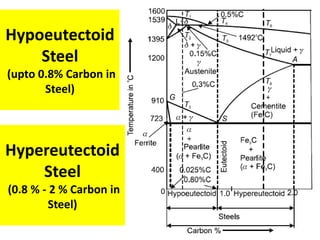
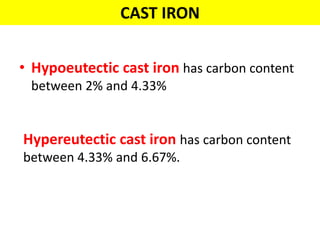
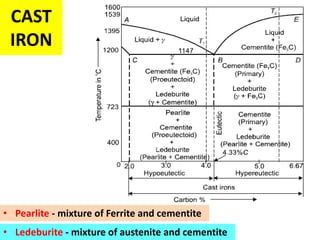
The document discusses the constitution of alloys in engineering metallurgy, explaining the necessity of alloying to enhance properties like strength and toughness. It covers various types of solid solutions (substitutional and interstitial), phase diagrams, cooling curves, and the Gibbs phase rule, illustrating the solidification processes of pure metals and binary alloys. Additionally, it details the reactions in the iron-carbon system and categorizes steels and cast irons based on carbon content.