Embed presentation
Downloaded 315 times


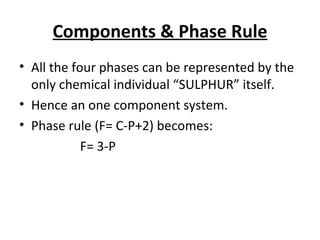

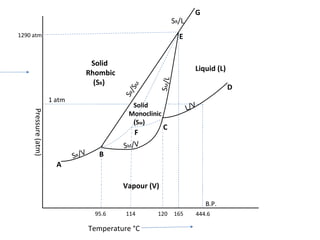
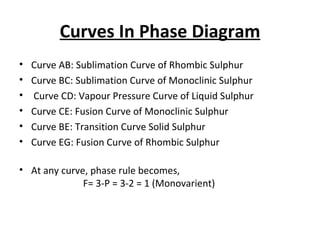
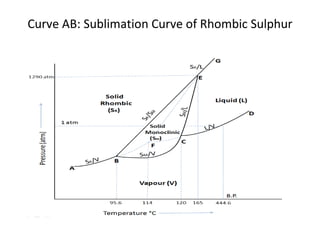
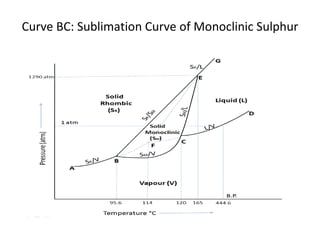
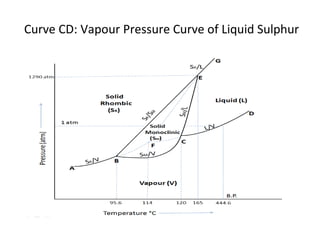
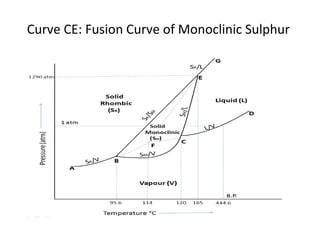

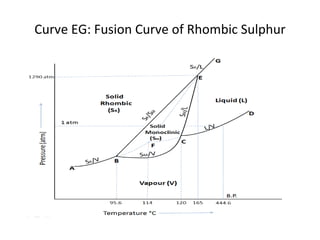
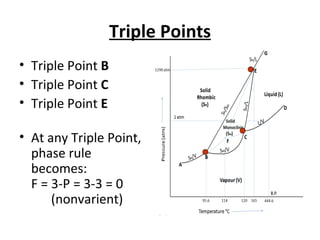

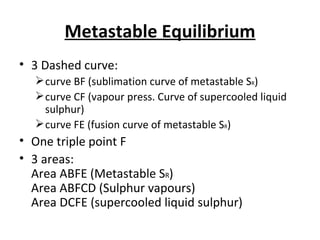


The document summarizes the sulphur system phase diagram. It describes that sulphur can exist in four phases: rhombic solid, monoclinic solid, liquid, and vapor. The phase diagram shows the phase boundary curves between these different sulphur phases. It includes the sublimation, vapor pressure, fusion/melting, and transition curves. The phase diagram areas represent stable regions where the sulphur can exist as rhombic solid, monoclinic solid, liquid, or vapor. Triple points occur where three phases meet in equilibrium. The diagram also shows a region of metastable equilibrium with supercooled liquid sulfur and metastable rhombic solid phases.















