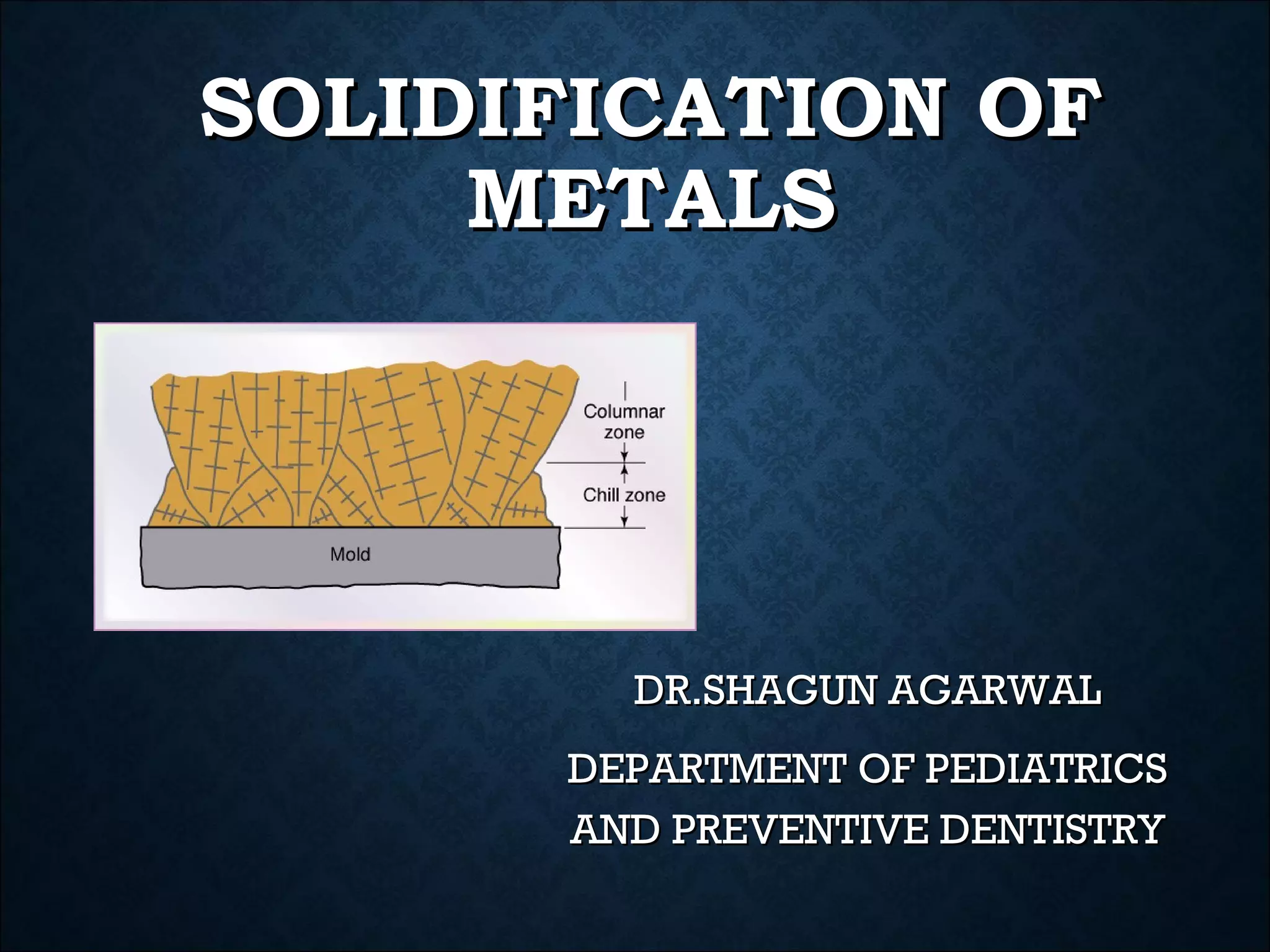
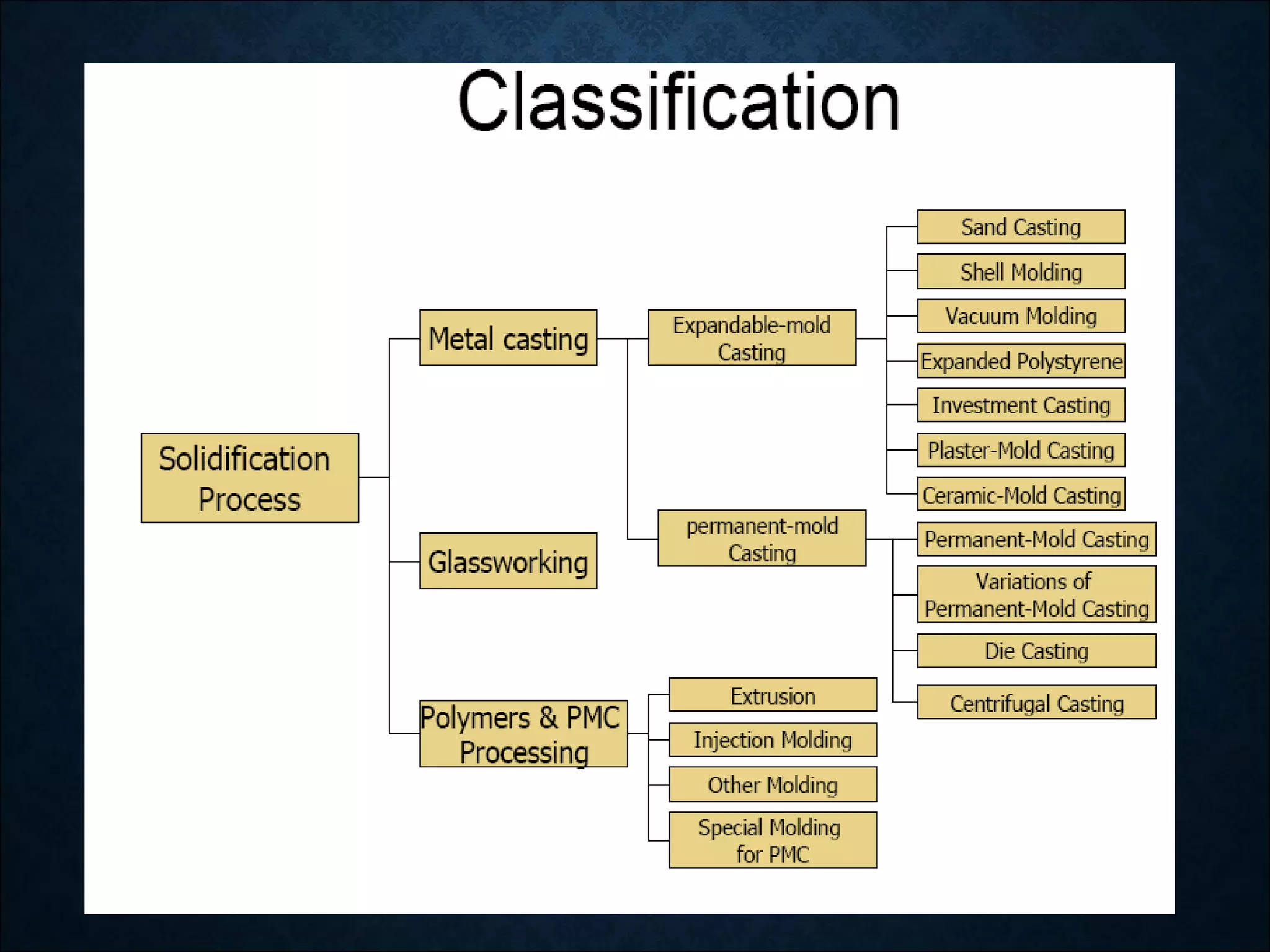
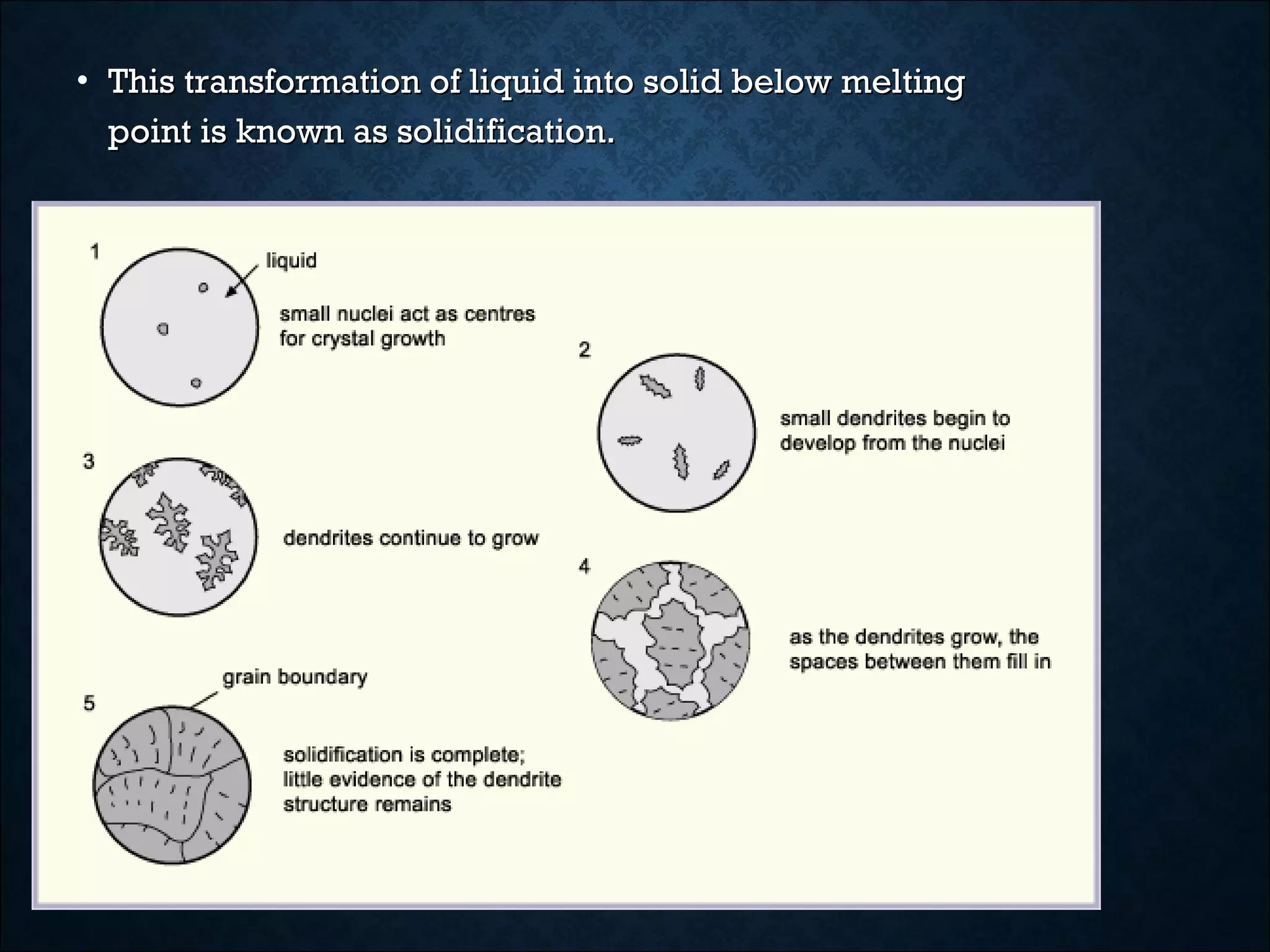
This document discusses the solidification of metals. It begins with an introduction to metals and their importance in dentistry. It then covers the classification of metals and their properties like conductivity. The document discusses the history of metals and how solidification occurs through nucleation and crystal growth below the melting point. It provides examples of solidification patterns in metals like steel and how properties like carbon content affect the patterns.