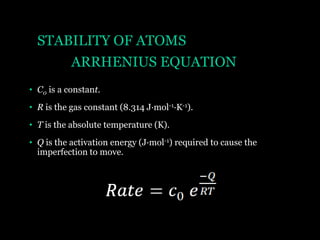
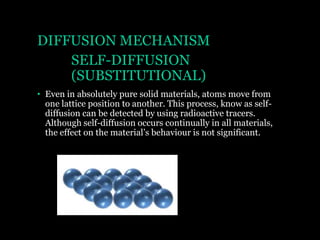
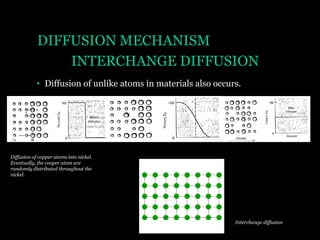
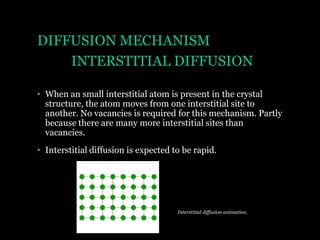
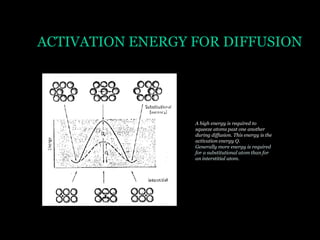
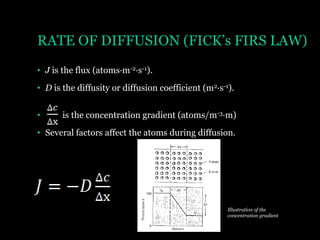
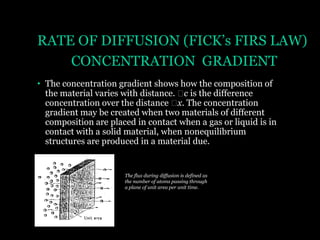
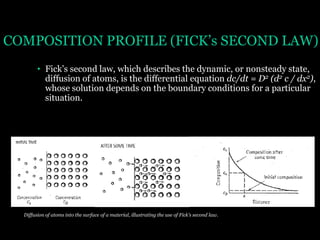
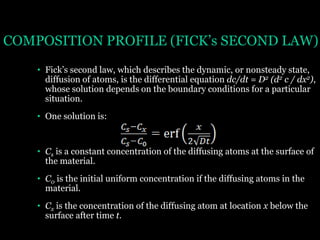
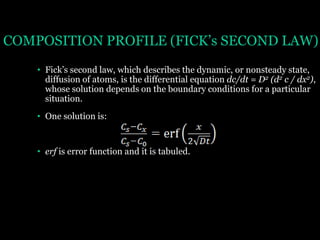
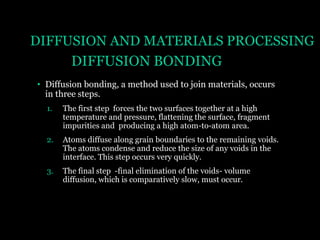
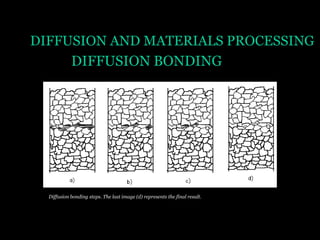
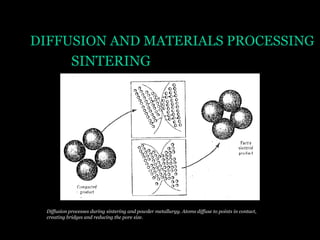
This document discusses the process of diffusion in materials. It defines diffusion as the movement of atoms within a material driven by concentration gradients. There are four main mechanisms of diffusion: self-diffusion, interchange diffusion, vacancy diffusion, and interstitial diffusion. Diffusion is thermally activated and follows the Arrhenius equation, requiring atoms to overcome an activation energy barrier. Fick's first law describes the flux of atoms down a concentration gradient, while Fick's second law models the change in concentration over time. Diffusion plays a key role in processes like grain growth, diffusion bonding, and sintering of materials.