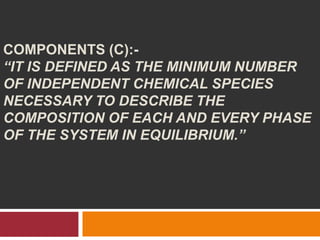
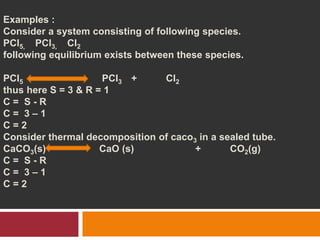
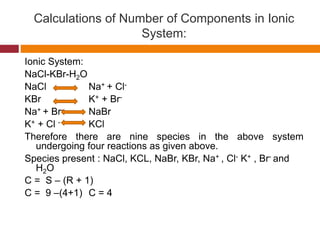
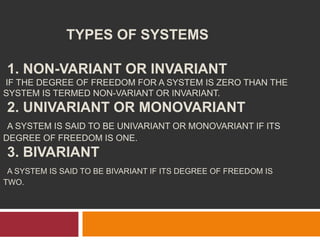
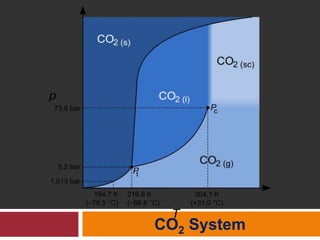
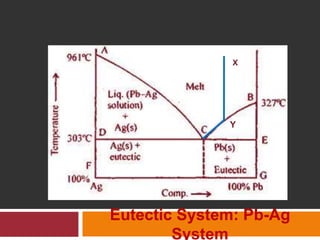
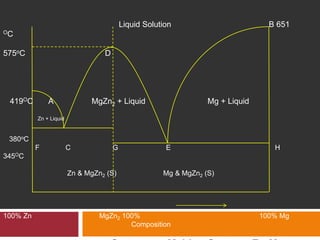
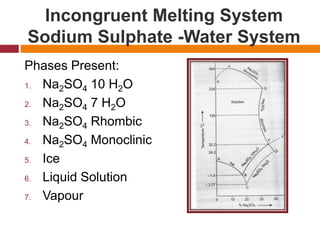
The document explains Gibbs' Phase Rule, which describes the relationship between the number of components, phases, and degrees of freedom in heterogeneous systems at equilibrium. It details the definitions of phases and components, provides examples, and illustrates how to calculate degrees of freedom using the equation f = c - p + 2. Additionally, the document discusses phase diagrams, cooling curves, and applications of the phase rule, including lyophilization.