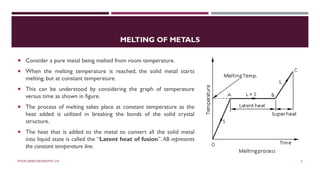
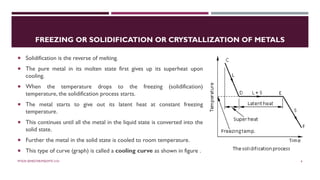
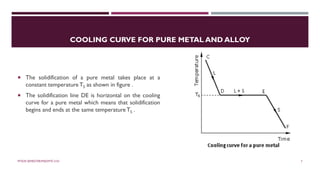
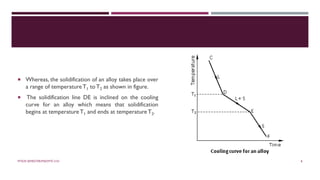
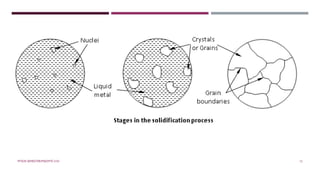
Chapter 2 discusses the mechanisms of solidification and phase diagrams, focusing on the processes of homogeneous and heterogeneous nucleation, crystal growth, and the properties of solid solutions. It emphasizes that solidification transforms liquid metal into solid, influenced by cooling rates and mold types, while detailing phases such as freezing temperature and latent heat of fusion. Key concepts include nucleation, crystal growth, and the formation of grain structures in metals and alloys.