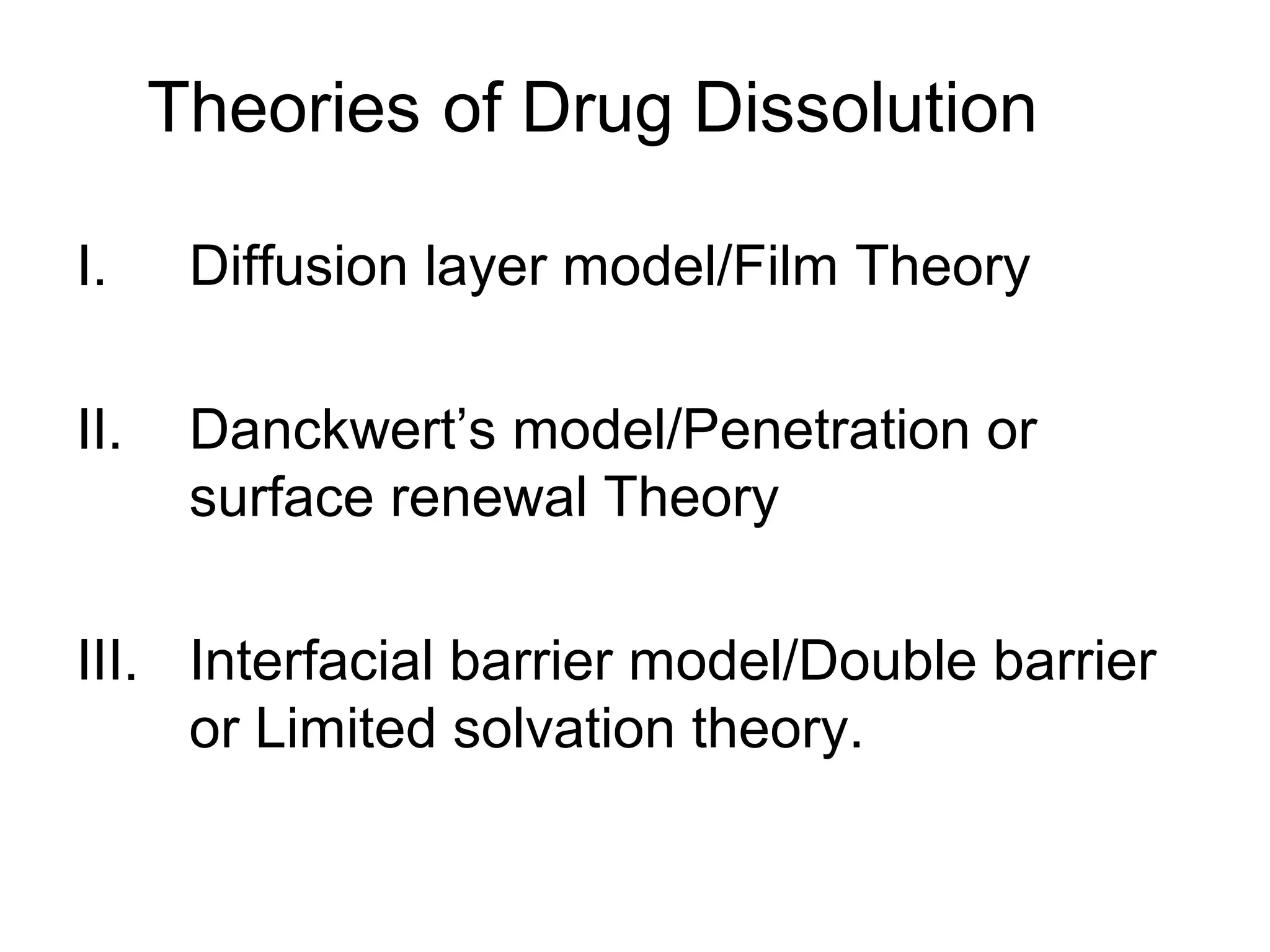
The document discusses three main theories of drug dissolution:
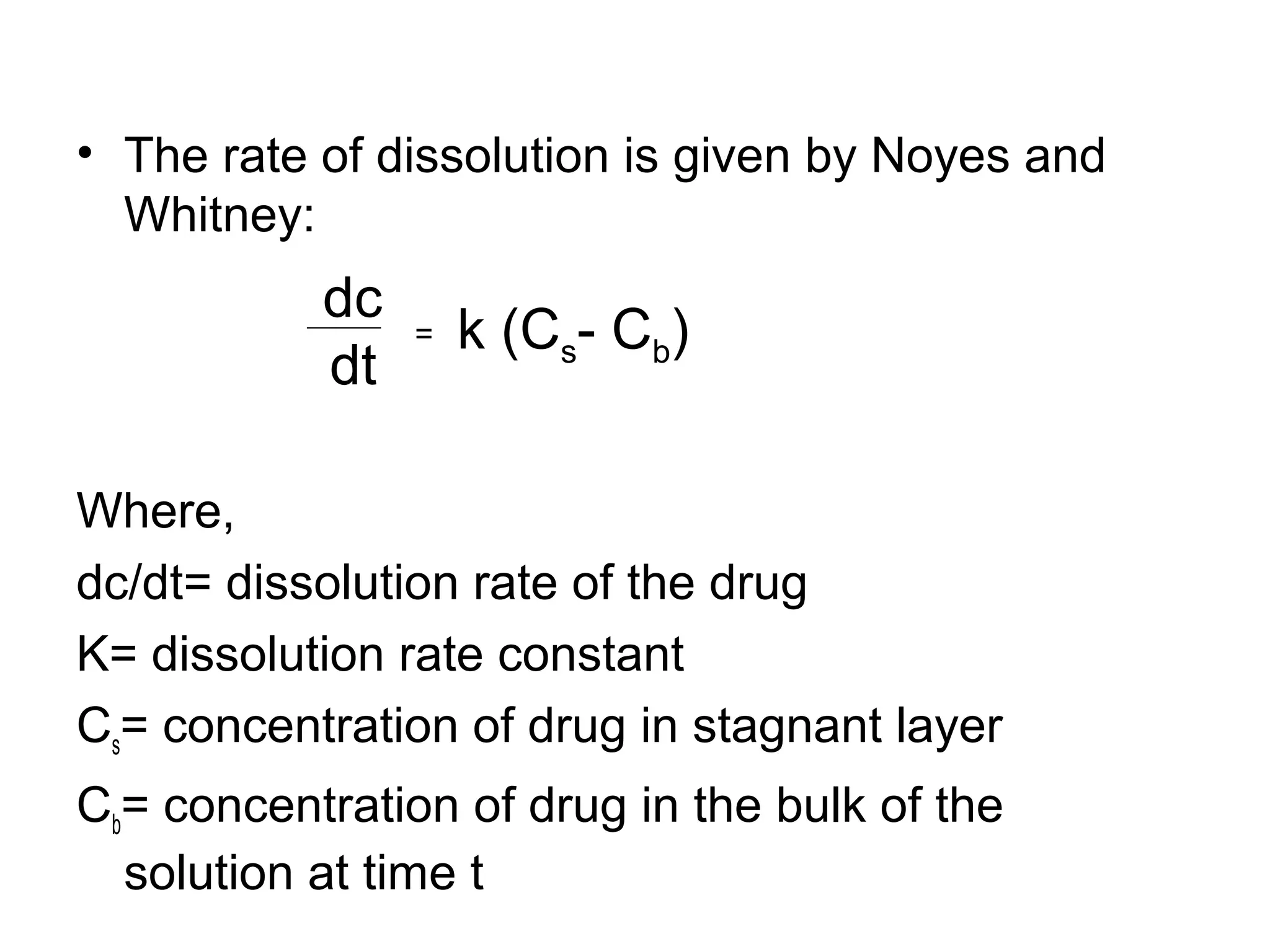
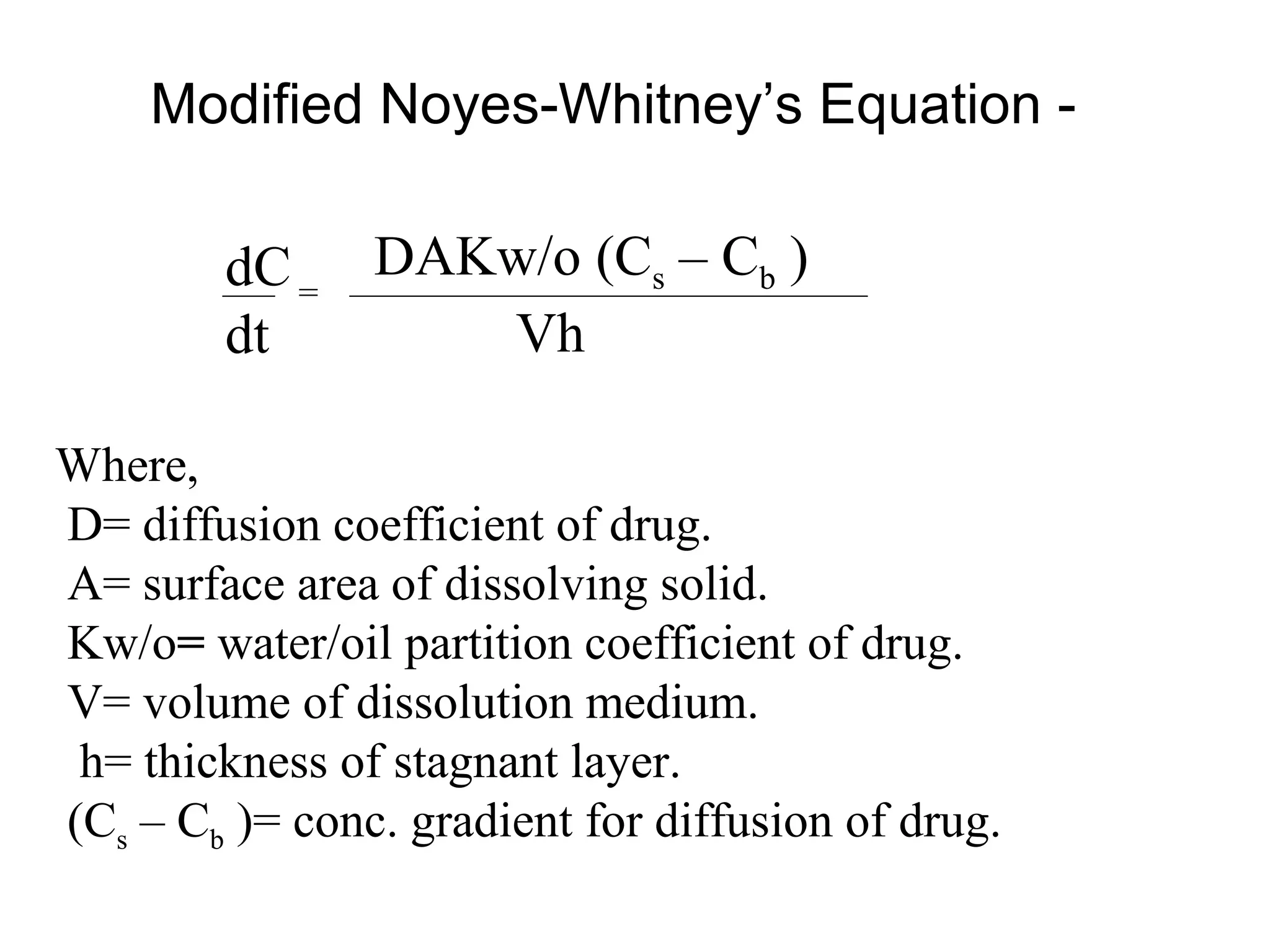
1) Diffusion layer/film theory which describes dissolution as a two step process of drug dissolving from the solid to form a saturated film and then diffusing out of the film. The rate of dissolution is given by the Noyes-Whitney equation.
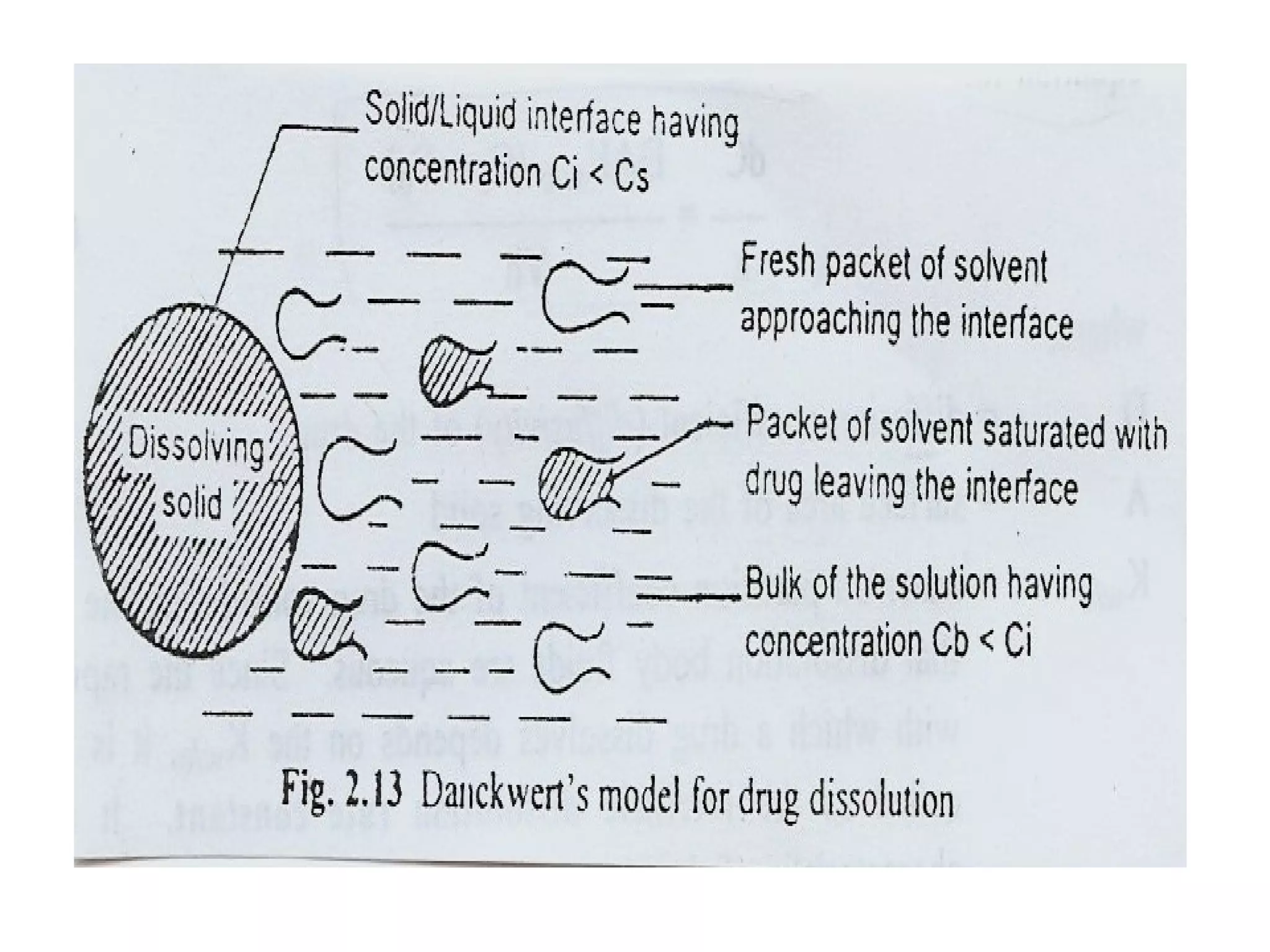
2) Danckwert's/surface renewal theory which accounts for eddies in the solution exposing new surface areas for dissolution. The rate is expressed as the product of the surface renewal rate and concentration gradient.
3) Interfacial barrier model which assumes the reaction at the solid surface is slower than diffusion, making interfacial transport the rate limiting step described by another equation.