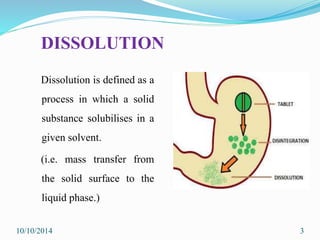
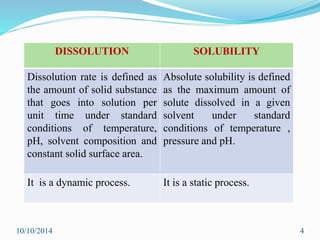
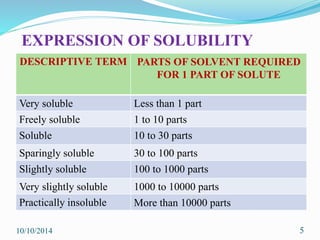
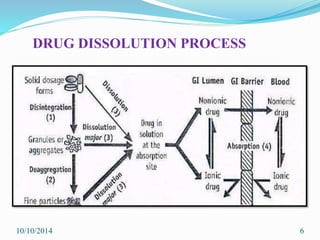
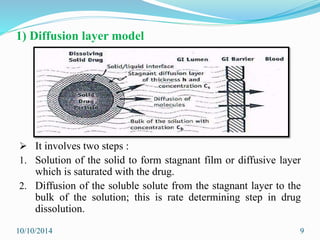
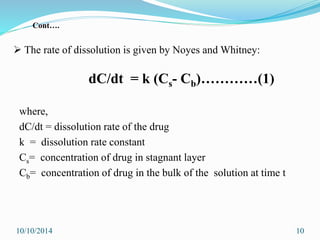
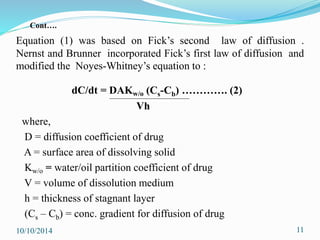
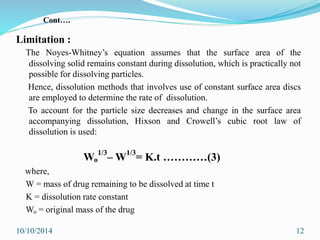
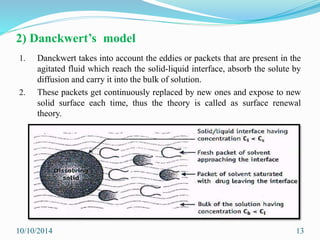
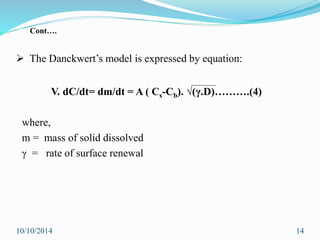
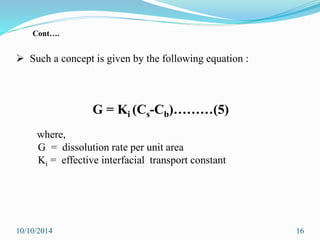
The document presents an overview of dissolution in pharmaceutics, defining it as the process of a solid substance dissolving in a solvent and discussing key concepts such as dissolution rate and solubility. It outlines the drug dissolution process, detailing the steps involved in releasing a drug from a tablet and describes three theories of dissolution: diffusion layer model, Danckwert’s model, and interfacial barrier model. The document also provides mathematical equations related to these theories and includes references for further reading.