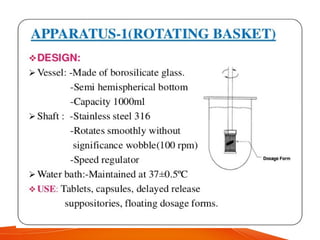
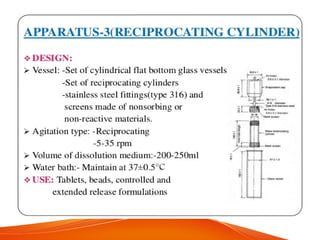
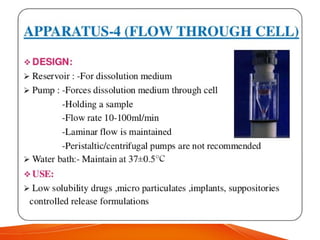
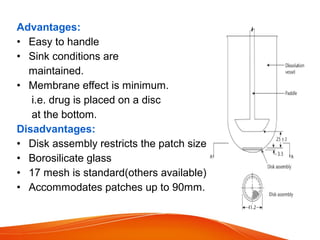
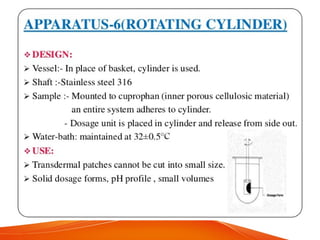
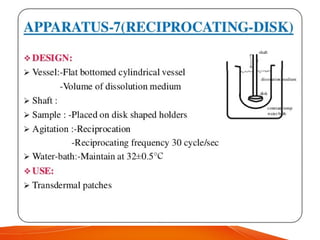
The document discusses invitro dissolution testing of drugs. It defines dissolution rate and invitro dissolution tests as tests used to measure the rate and extent of dissolution of a drug from its formulation under specified conditions. Key factors in designing dissolution tests include the apparatus used, dissolution medium properties, and process parameters. Common apparatuses include basket, paddle, reciprocating cylinder, and flow-through cell methods. Dissolution testing provides important information on a drug's in vivo performance and quality control.