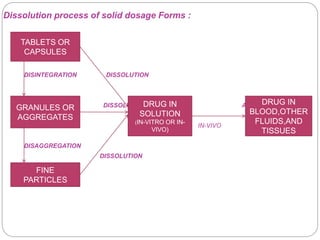
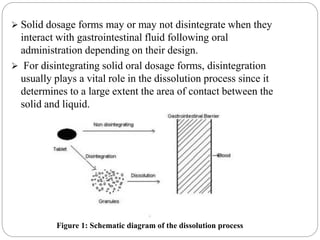
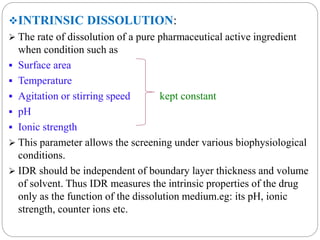
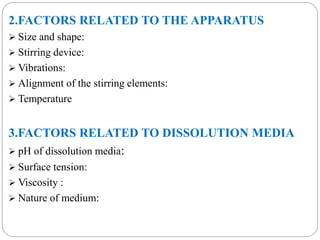
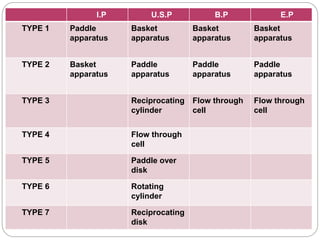
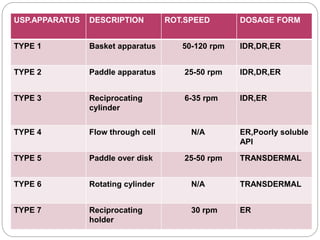
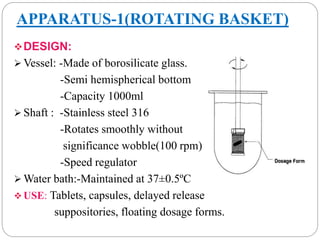
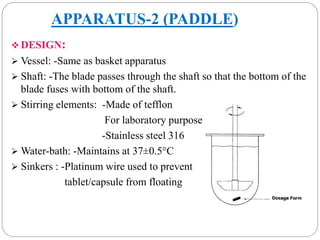
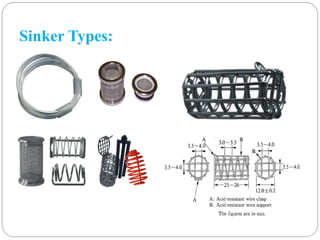
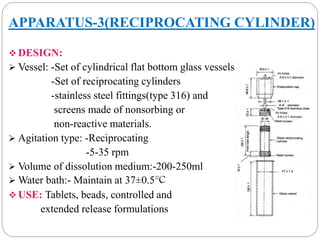
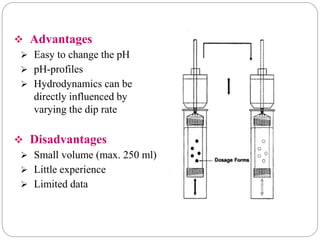
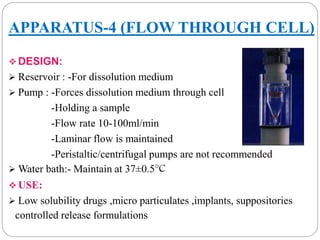
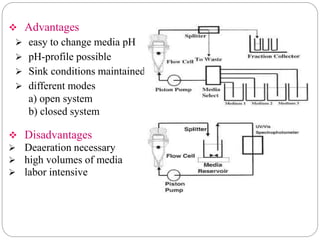
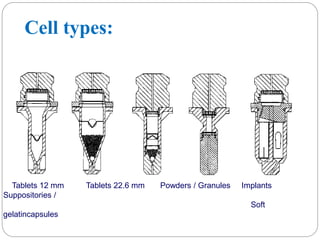
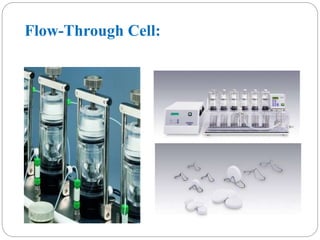
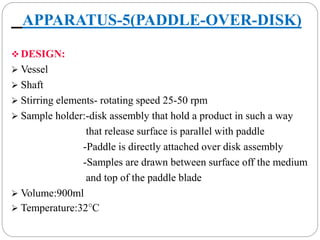
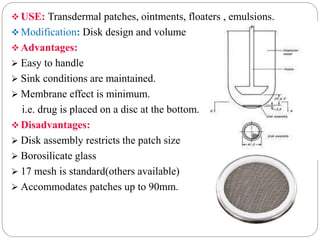
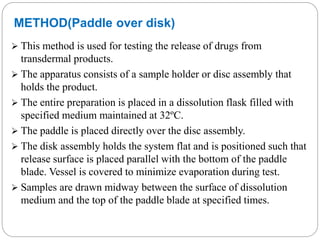
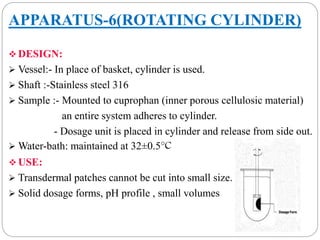
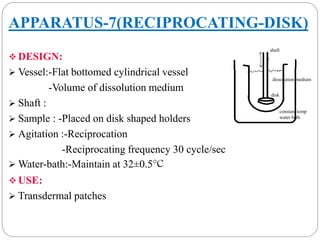
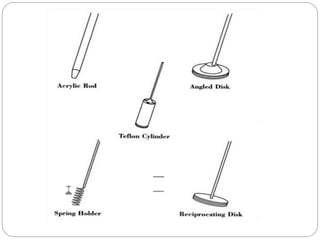
This document provides an overview of dissolution testing and the factors that influence drug dissolution. It defines dissolution and describes the intrinsic dissolution process. It also discusses the various apparatus used for dissolution testing according to pharmacopeial specifications, including the basket, paddle, reciprocating cylinder, and flow-through cell. The key factors affecting dissolution are also summarized, such as drug properties, apparatus parameters, and media properties.