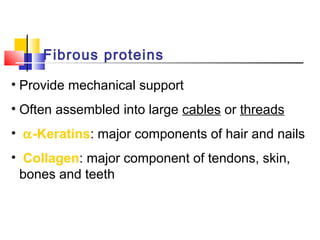
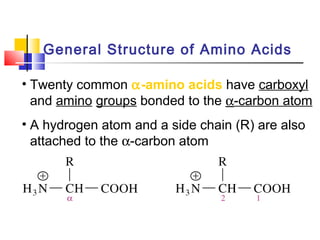
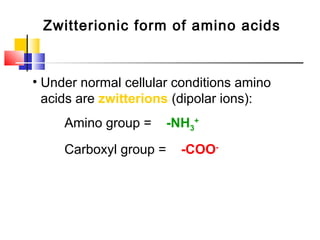
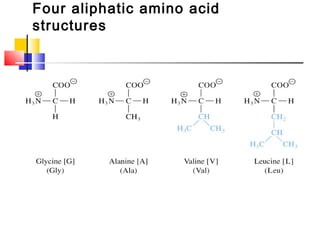
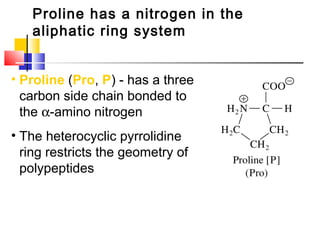
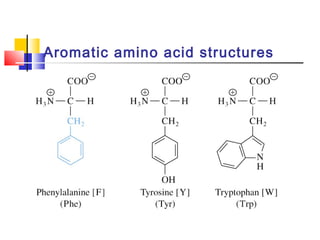
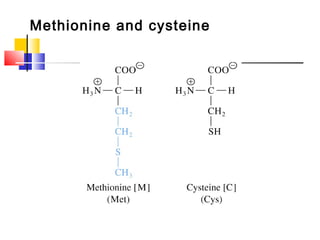
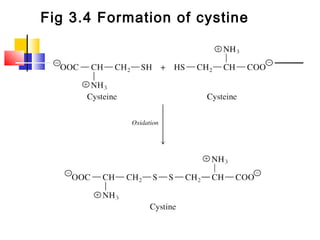
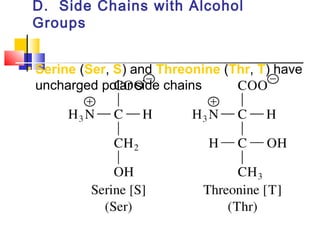
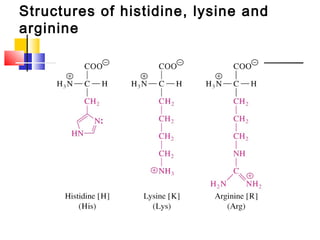
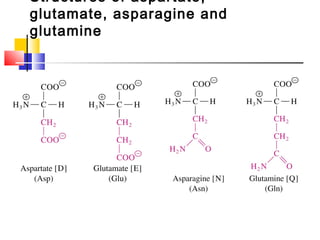
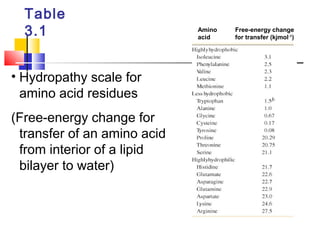
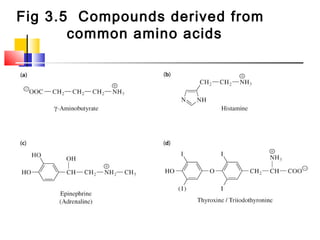
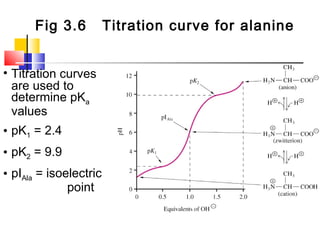
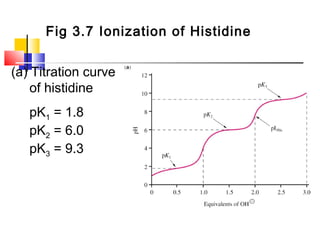
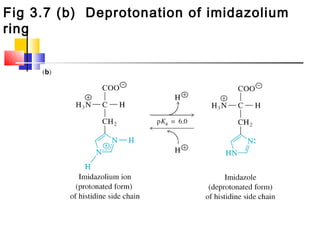
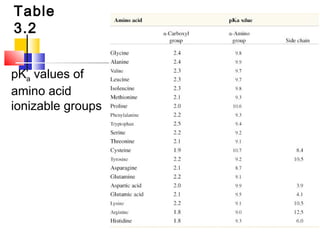
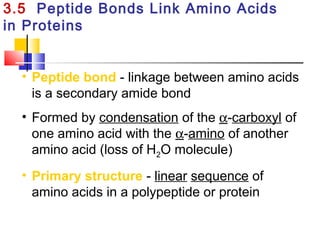
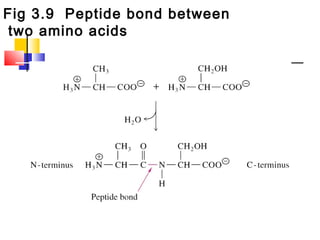
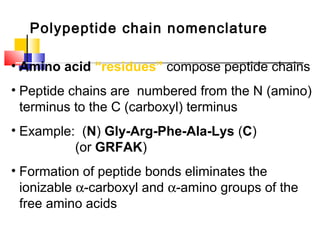
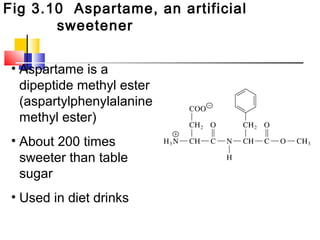
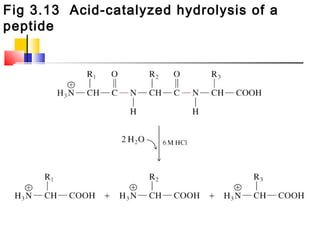
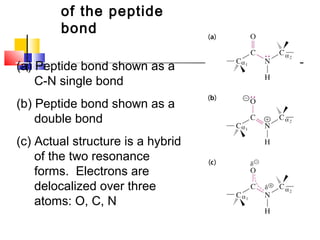
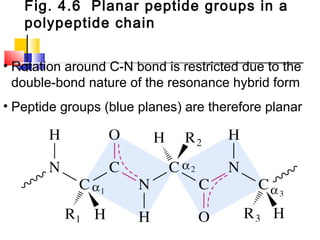
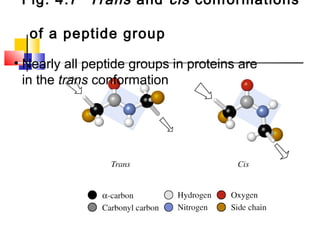
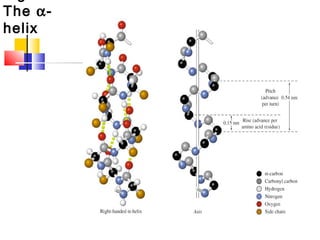
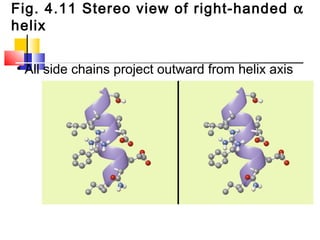
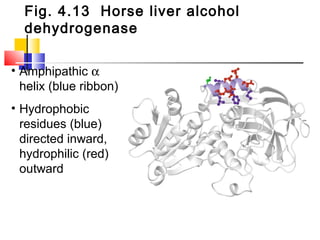
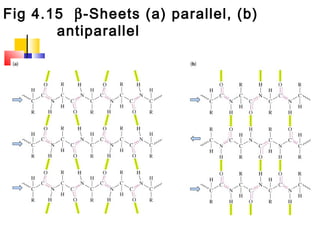
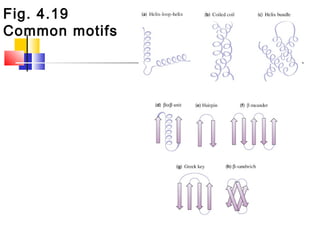
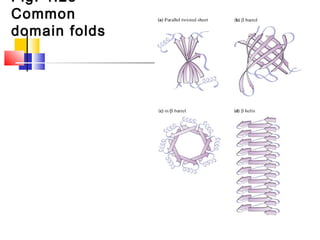
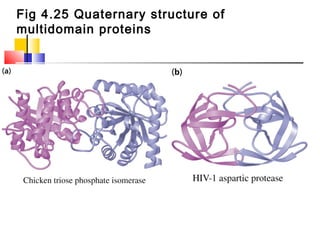
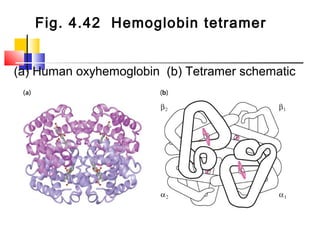
This document discusses the primary, secondary, tertiary, and quaternary structure of proteins. It begins by describing the important biological functions of proteins and the general structures of globular and fibrous proteins. It then discusses the structures of amino acids and how peptide bonds link amino acids into polypeptide chains. The levels of protein structure are introduced, including the alpha helix and beta sheet secondary structures, tertiary folding of polypeptide chains, and arrangement of subunits in quaternary structure. Common protein domains and motifs are also illustrated.