The document discusses the Biopharmaceutical Classification System (BCS), established in 1995, which categorizes drug substances based on solubility, permeability, and dissolution rate to predict in vivo pharmacokinetics. It outlines four classes of drugs based on these criteria, each with specific characteristics, applications, and examples. Additionally, it covers the concept of biowaivers, permitting exemptions from human bioequivalence studies under certain conditions.
![Biopharmaceutical Classification
System [BCS]
1
Department of Pharmaceutics | Sagar savale
Mr. Sagar Kishor Savale
[Department of Pharmaceutics]
avengersagar16@gmail.com](https://image.slidesharecdn.com/biopharmaceuticalclassificationystembcs-160328061345/75/Bio-pharmaceutical-classification-System-BCS-1-2048.jpg)
![CONTENT
INTRODUCTION
FACTOR AFFECTING ON BIOPHARMACEUTICAL CLASSIFICATION SYSTEM
BIOPHARMACEUTICAL CLASSIFICATION SYSTEM [CLASSES]
APPLICATION OF BIOPHARMACEUTICAL CLASSIFICATION SYSTEM
CLASS BOUNDARIES
BIOWAIVER
CRITERIA OF BIOWAIVER
CONCLUSION
REFERENCES
2](https://image.slidesharecdn.com/biopharmaceuticalclassificationystembcs-160328061345/75/Bio-pharmaceutical-classification-System-BCS-2-2048.jpg)
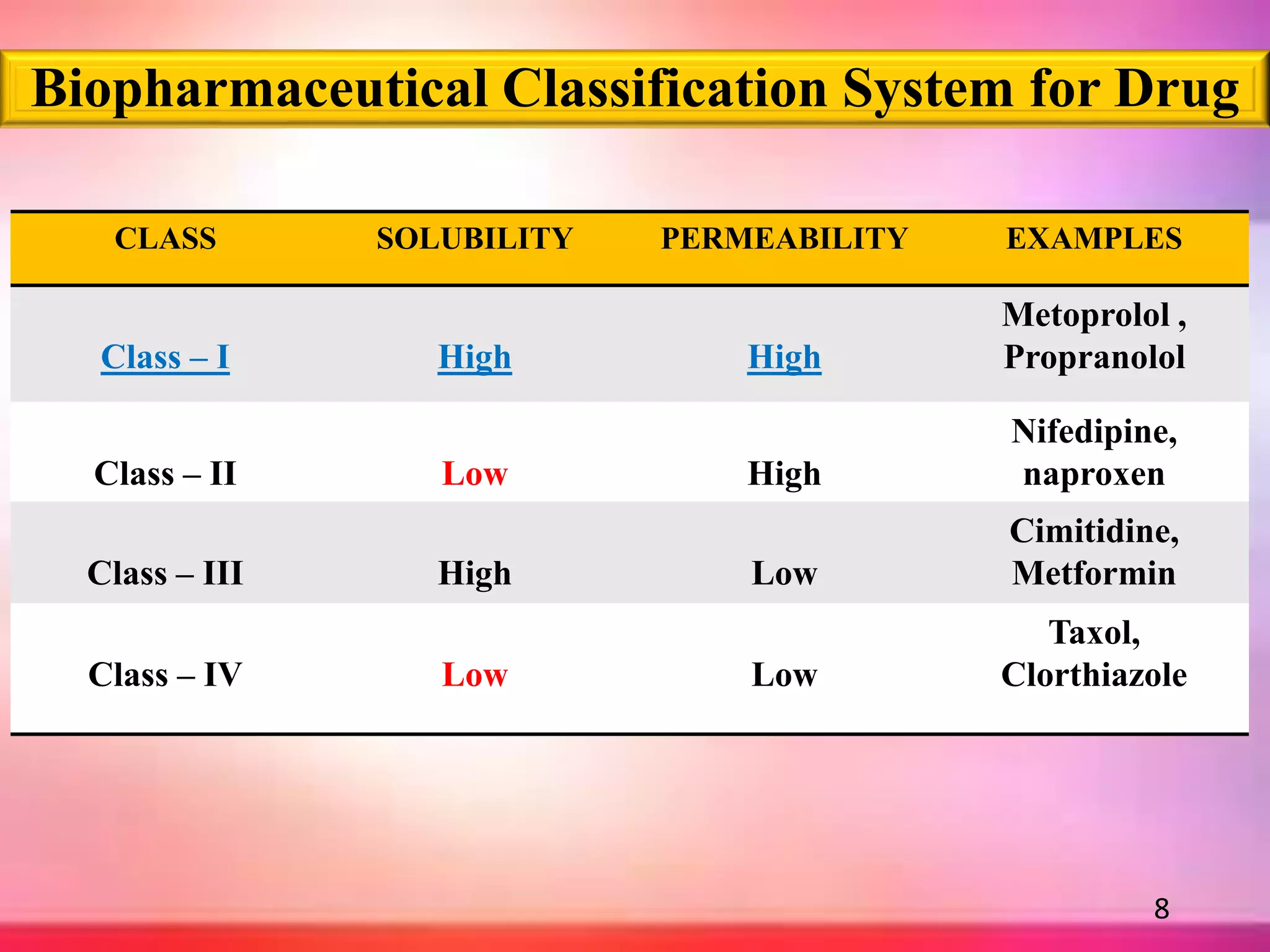
![BIOPHARMACEUTICAL CLASSIFICATION SYSTEM [BCS]
The Biopharmaceutical Classification System was first developed by in 1995,
by Amidon et al & his colleagues.
Definition:
“The Biopharmaceutical Classification System is a scientific
framework for classifying a drug substance based on its aqueous solubility &
intestinal permeability & dissolution rate”.
To saved time fast screening is required so drug substances are classified
on basis of solubility and permeability. This classification is called
Biopharmaceutical Classification System.
3](https://image.slidesharecdn.com/biopharmaceuticalclassificationystembcs-160328061345/75/Bio-pharmaceutical-classification-System-BCS-3-2048.jpg)
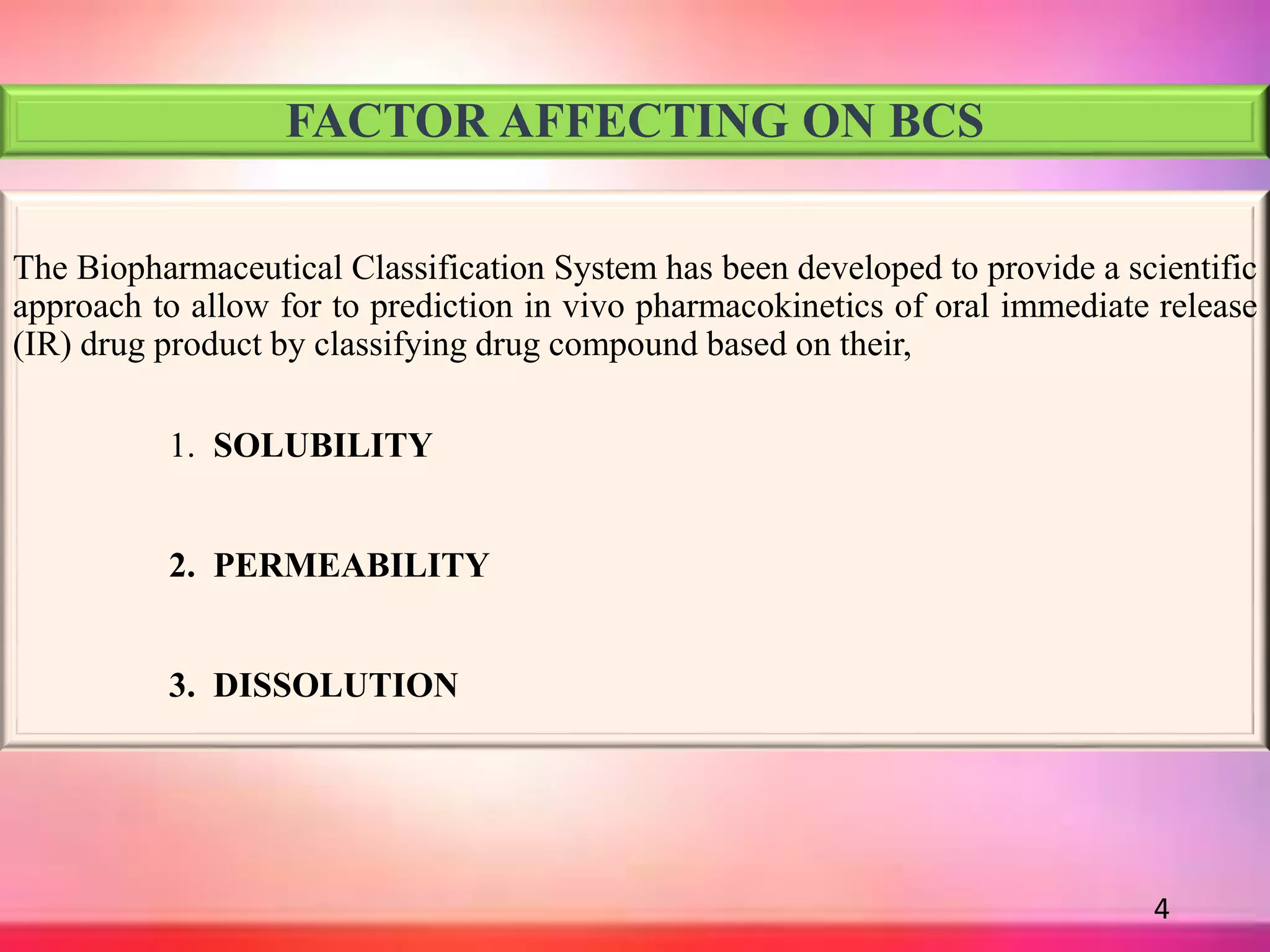


![DISSOLUTION
It is process in which solid substance solubilises in given solvent i.e mass
transfer from solid surface to liquid phase.
Using USP apparatus I at 100 rpm or USP apparatus II at 50 rpm .
Dissolution Media [900 ml],
1. 0.1 N HCl or simulated gastric fluid (pH 1.2) without enzyme.
2. pH 4.5 buffer & pH 6.8 buffer.
3. Simulated intestinal fluid without enzyme.
7](https://image.slidesharecdn.com/biopharmaceuticalclassificationystembcs-160328061345/75/Bio-pharmaceutical-classification-System-BCS-7-2048.jpg)