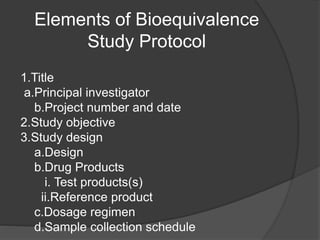
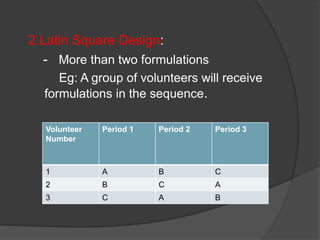
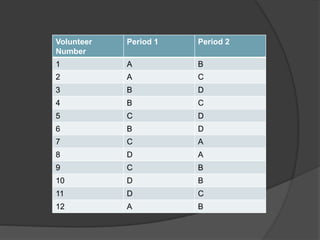
The document outlines the key elements of a bioequivalence study protocol, including:
1. The objective is to show that the test and reference drug products have similar bioavailability when administered at the same dose.
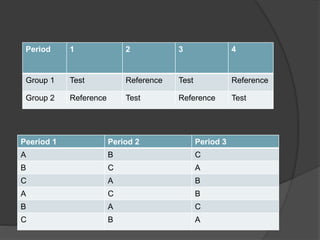
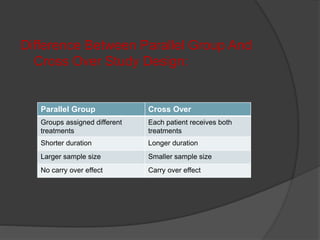
2. The study design typically involves both fasting and fed conditions, uses a crossover or parallel group design, and involves pharmacokinetic analysis of blood samples taken over time.
3. Subject selection criteria aim to minimize variability through screening and exclusion of those with health issues that could impact drug absorption.