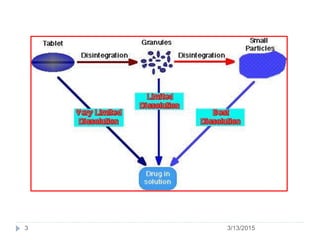
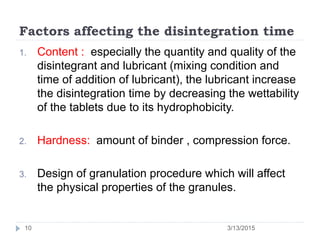
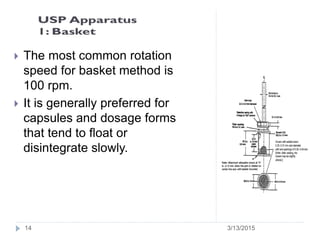
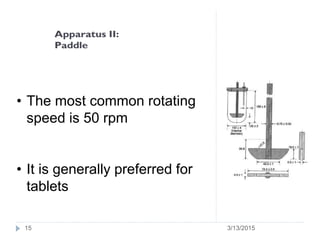
The document discusses disintegration and dissolution tests for tablets. The disintegration test uses 6 glass tubes with tablets placed in baskets that move up and down in fluid to check if tablets break down within a specified time. Factors like hardness and excipients affect disintegration time. The dissolution test uses apparatus like baskets or paddles that rotate tablets in fluid to determine the drug release rate over time and ensure bioequivalence. Proper conditions like sink volume and agitation are needed. Dissolution is important to show drug availability and batch consistency.