Std XI-Chem-Ch1-Concepts-Chemical-reactions-Stoichiometry
•Download as PPTX, PDF•
3 likes•868 views
This document provides an overview of chemical reactions and stoichiometry for a Grade 11 chemistry class. It discusses the balanced chemical equation for the formation of water, and how balancing the equation ensures the conservation of mass. Stoichiometry is introduced as the process of making calculations based on balanced chemical equations. Limiting and excess reactants are defined, with an example showing how to determine which reactant is limiting based on the mole ratios provided and actual amounts available.
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Class 10 Chemistry Chapter 1 chemical reactions and equations

Class 10 Chemistry Chapter 1 chemical reactions and equations
Science ppt 10th class chemical reactions by Jeyasuriya

Science ppt 10th class chemical reactions by Jeyasuriya
Class 10 l Science l Chemistry l Lesson 1: Chemical equations and reactions

Class 10 l Science l Chemistry l Lesson 1: Chemical equations and reactions
Viewers also liked
Viewers also liked (20)
Std XI-Chem-Ch1-Concepts-Isotopes-Atomic-mass-Molecular-mass

Std XI-Chem-Ch1-Concepts-Isotopes-Atomic-mass-Molecular-mass
Similar to Std XI-Chem-Ch1-Concepts-Chemical-reactions-Stoichiometry
Similar to Std XI-Chem-Ch1-Concepts-Chemical-reactions-Stoichiometry (20)
2011 topic 01 lecture 3 - limiting reactant and percent yield

2011 topic 01 lecture 3 - limiting reactant and percent yield
IB Chemistry Limiting, Excess, Theoretical and Percentage Yield

IB Chemistry Limiting, Excess, Theoretical and Percentage Yield
More from Gurudatta Wagh
More from Gurudatta Wagh (17)
Recently uploaded
This presentation was provided by William Mattingly of the Smithsonian Institution, during the closing segment of the NISO training series "AI & Prompt Design." Session Eight: Limitations and Potential Solutions, was held on May 23, 2024.Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"

Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"National Information Standards Organization (NISO)
Recently uploaded (20)
Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"

Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"
Benefits and Challenges of Using Open Educational Resources

Benefits and Challenges of Using Open Educational Resources
The Art Pastor's Guide to Sabbath | Steve Thomason

The Art Pastor's Guide to Sabbath | Steve Thomason
Salient features of Environment protection Act 1986.pptx

Salient features of Environment protection Act 1986.pptx
Students, digital devices and success - Andreas Schleicher - 27 May 2024..pptx

Students, digital devices and success - Andreas Schleicher - 27 May 2024..pptx
The Benefits and Challenges of Open Educational Resources

The Benefits and Challenges of Open Educational Resources
INU_CAPSTONEDESIGN_비밀번호486_업로드용 발표자료.pdf

INU_CAPSTONEDESIGN_비밀번호486_업로드용 발표자료.pdf
Matatag-Curriculum and the 21st Century Skills Presentation.pptx

Matatag-Curriculum and the 21st Century Skills Presentation.pptx
MARUTI SUZUKI- A Successful Joint Venture in India.pptx

MARUTI SUZUKI- A Successful Joint Venture in India.pptx
Application of Matrices in real life. Presentation on application of matrices

Application of Matrices in real life. Presentation on application of matrices
Jose-Rizal-and-Philippine-Nationalism-National-Symbol-2.pptx

Jose-Rizal-and-Philippine-Nationalism-National-Symbol-2.pptx
slides CapTechTalks Webinar May 2024 Alexander Perry.pptx

slides CapTechTalks Webinar May 2024 Alexander Perry.pptx
Std XI-Chem-Ch1-Concepts-Chemical-reactions-Stoichiometry
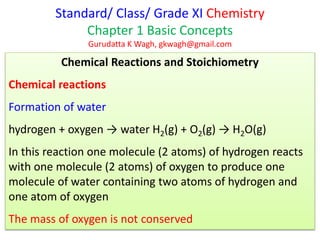
- 1. Standard/ Class/ Grade XI Chemistry Chapter 1 Basic Concepts Gurudatta K Wagh, gkwagh@gmail.com Chemical Reactions and Stoichiometry Chemical reactions Formation of water hydrogen + oxygen → water H2(g) + O2(g) → H2O(g) In this reaction one molecule (2 atoms) of hydrogen reacts with one molecule (2 atoms) of oxygen to produce one molecule of water containing two atoms of hydrogen and one atom of oxygen The mass of oxygen is not conserved
- 2. So we have to balance the equation, 2H2(g) + O2(g) → 2H2O(g) Let us now consider the molar mass, 2 X 2.02 g + 1 X 32 g → 2 (2 X 1.01 + 16) g 4.04 g + 32 g → 2 (2.02 + 16) g 36.04 g → 36.04 g Since the total mass of reactants = total mass of products, the molar mass is conserved Stoichiometry It is a process of making calculation based on formulae and balanced chemical equations Mass relationship The total mass of reactants must be equal to the total mass of products
- 3. Limiting and excess reactants In a chemical reaction the cost of the reactants plays an important role. To save on the cost the cheaper reactant is taken in excess and the costlier reactant is taken in lesser quantity. As the reaction begins the reactant that is taken in lesser quantity (costlier reactant) gets consumed first and the reaction stops. This reactant is called a limiting reactant. On the other hand the reactant taken in excess (cheaper reactant) does not get consumed fully and remains unreacted with the product.
- 4. E.g. 2Au + 3Cl2 → 2AuCl3 2 X 196.97 g + 3 X 35.45 X 2 → 2 (196.97 + 3X 35.45) 393.94 g + 212.7 g → 606.64 g Hence 606.64 g → 606.64 g molar ratio Au : Cl = 2 : 3 = 0.667 If 10 g each of Au and Cl2 is used Mole of Au = 10 g/ 196.97 = 0.0508 Mole of Cl2 = 10 g/ 70.90 = 0.141 Ratio of actual moles of Au : Cl2 = 0.0508 : 0.141 = 0.36 Since we get a ratio less than 1, it means Cl2 is the excess reactant and Au is the limiting reactant
- 5. Mole Au Mole Cl2 2 3 0.0508 ? (0.0508 X 3) ÷ 2 = 0.0762 It means 0.0508 mole of Au required 0.0762 mole of Cl2 Mole of Cl2 that remains unreacted = 0.141 – 0.0762 = 0.0648 Mass of Cl2 that has reacted = 0.0762 X 70.90 = 5.402 g Since 2 mole of Au produces 2 mole of AuCl3, hence 0.0508 mole of Au produces (0.0508 X 2) ÷ 2 = 0.0508 mole of AuCl3 Mass of AuCl3 produced = 0.0508 X 303.32 (1 AuCl3 = 303.32 g) = 15.409 g
