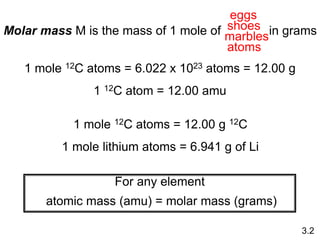
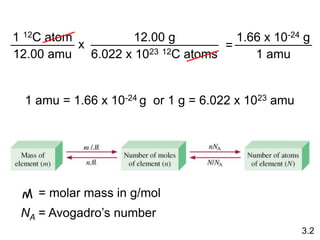
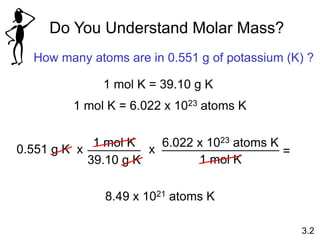
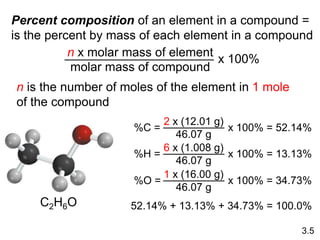
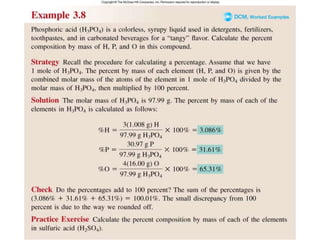
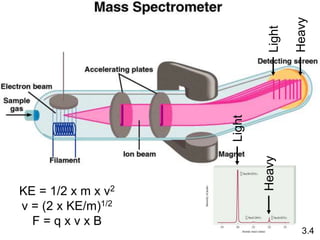
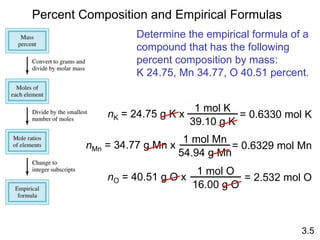
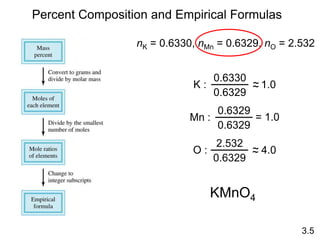
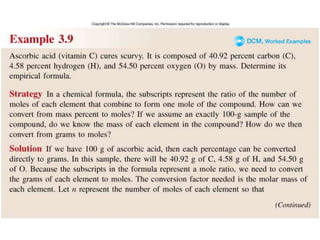
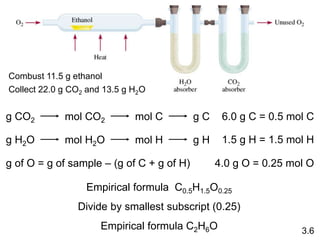
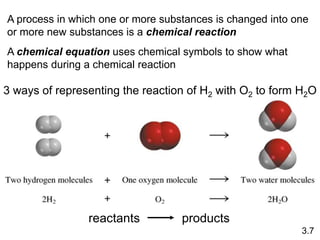
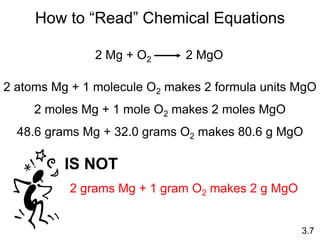
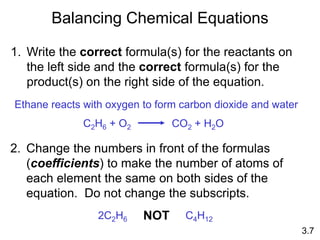
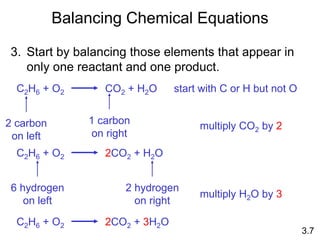
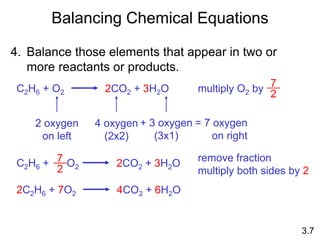
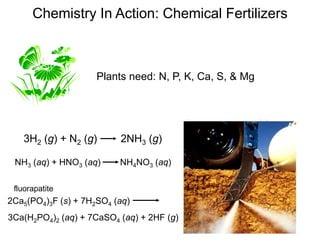
This document discusses mass relationships in chemical reactions, including:
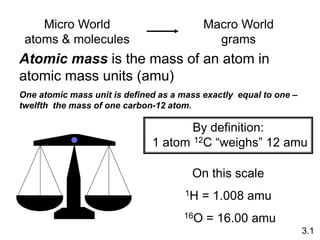
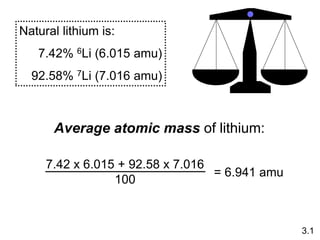
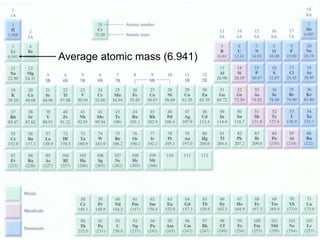
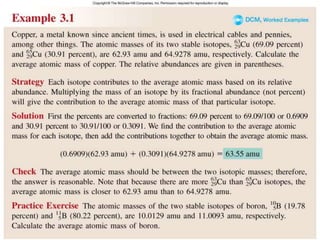
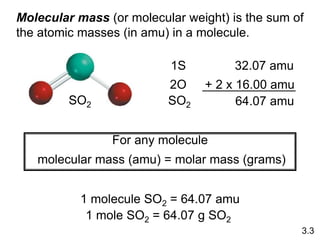
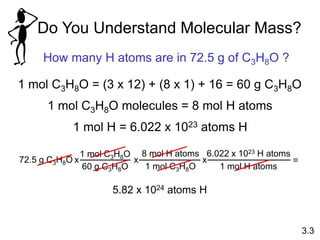
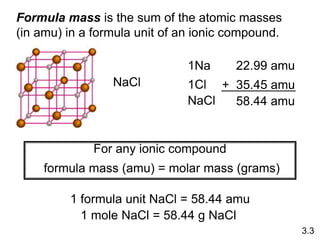
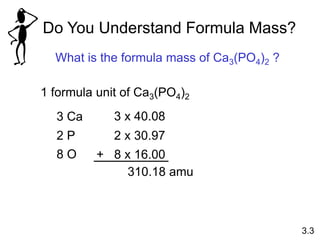
1) Atomic mass, molecular mass, molar mass, and formula mass. It defines the mole and Avogadro's number.
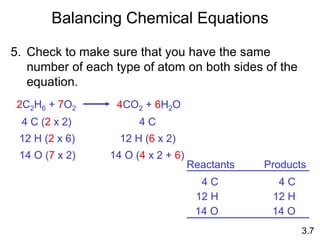
2) Chemical equations and how they are used to represent chemical reactions by balancing the atoms on each side.
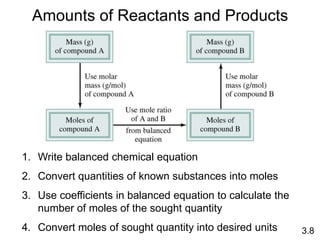
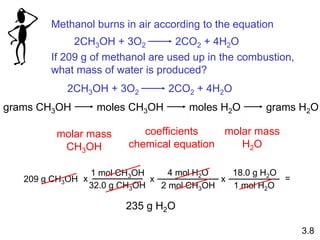
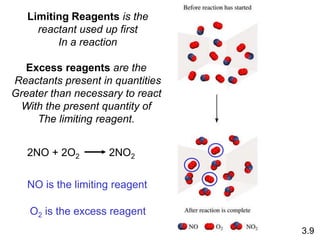
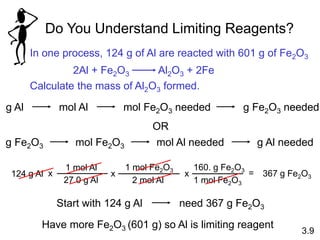
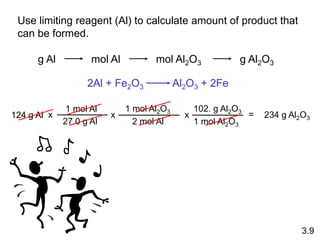
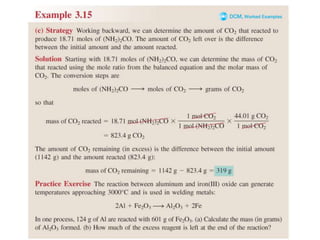
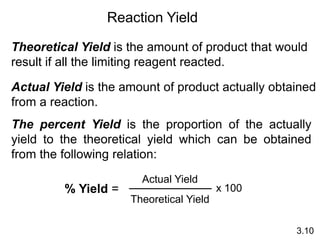
3) Calculations involving the amounts of reactants and products in chemical reactions, including limiting reagents.