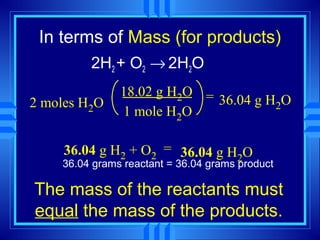
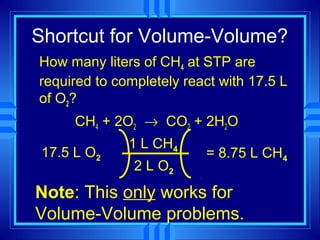
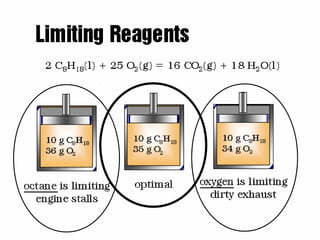
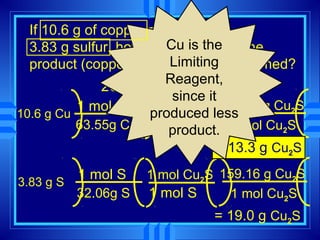
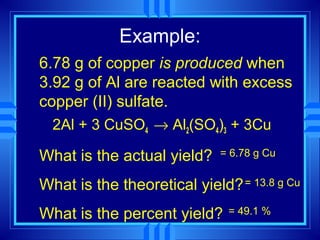
The document discusses stoichiometry, which is the calculation of quantities in chemical reactions based on a balanced equation. It explains how to interpret balanced equations in terms of particles, moles, mass, and gas volume. It also covers how to perform stoichiometric calculations using mole-mole, mole-mass, and volume-volume conversions based on molar ratios from balanced equations.