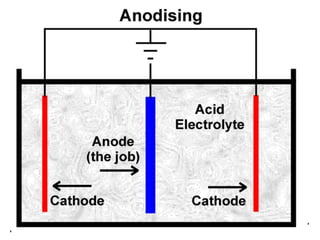
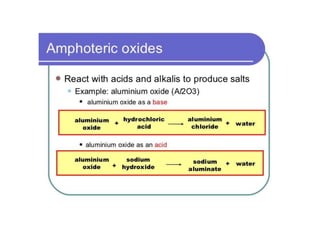
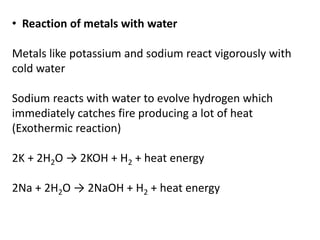
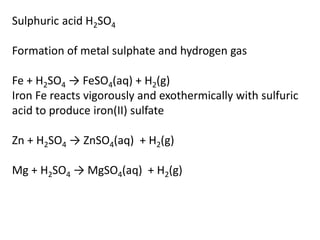
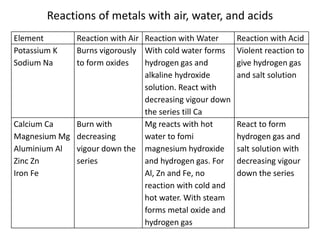
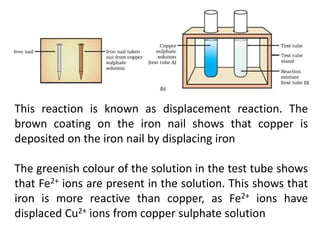
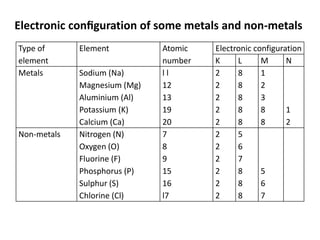
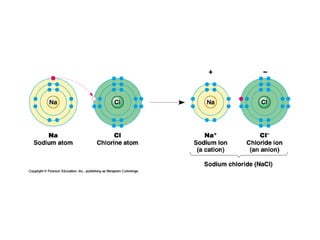
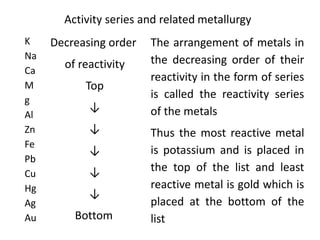
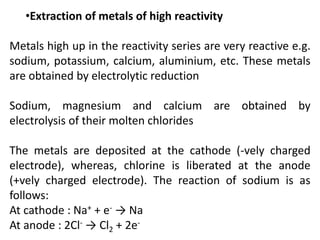
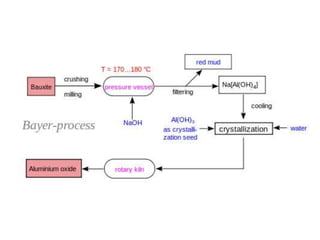
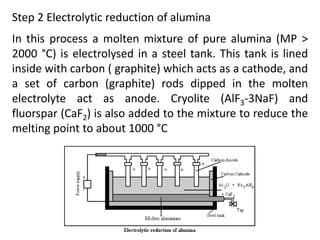
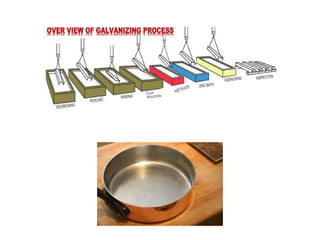
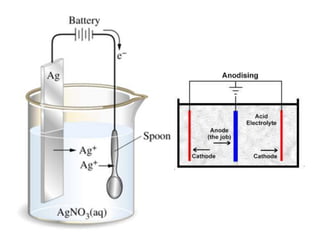
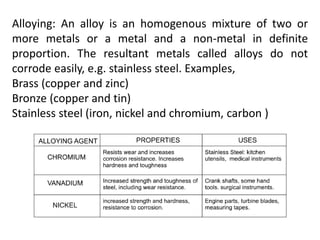
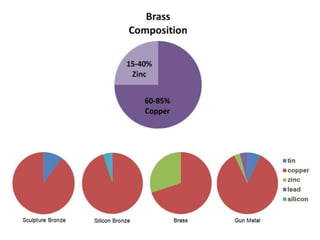
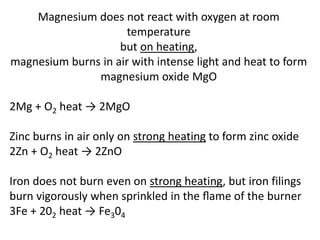This document provides an overview of metals and non-metals. It discusses their physical and chemical properties, including how metals react with oxygen, water, acids, and other substances. Metals are solid, malleable, ductile, and good conductors of heat and electricity. They form basic oxides and react vigorously with acids. Non-metals do not have these properties and are usually gases or solids. The document also covers extraction methods for metals and corrosion prevention.