The document discusses different types of chemical reactions:
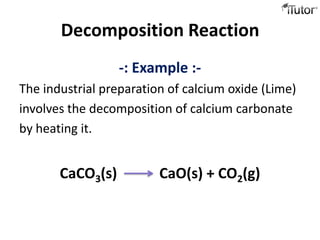
1) Decomposition reactions involve a single compound breaking down into simpler substances. An example is calcium carbonate decomposing to calcium oxide and carbon dioxide.
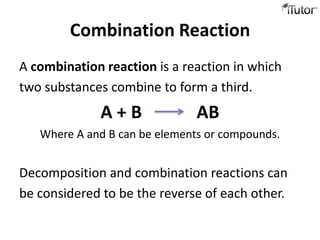
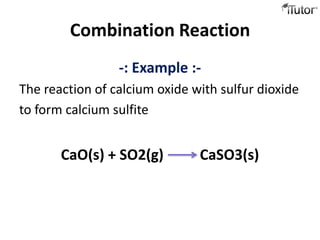
2) Combination reactions involve two substances combining to form a new compound. An example is calcium oxide and sulfur dioxide combining to form calcium sulfite.
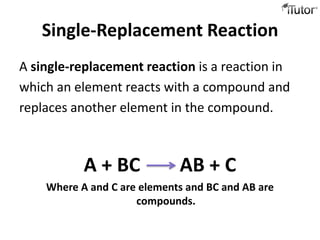
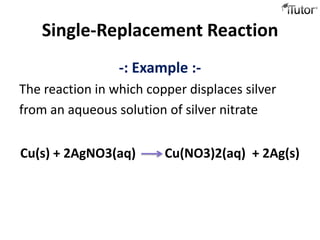
3) Single replacement reactions involve an element replacing another in a compound. An example is copper displacing silver from silver nitrate.
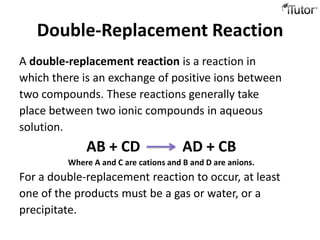
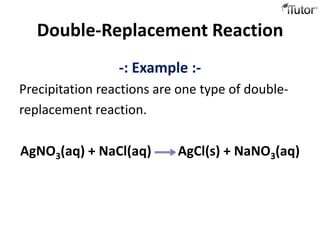
4) Double replacement reactions involve ion exchange between two compounds in solution and forming a precipitate. An example is silver nitrate and sodium chloride forming silver chloride and sodium nitrate precipitates.
5) Combustion reactions